
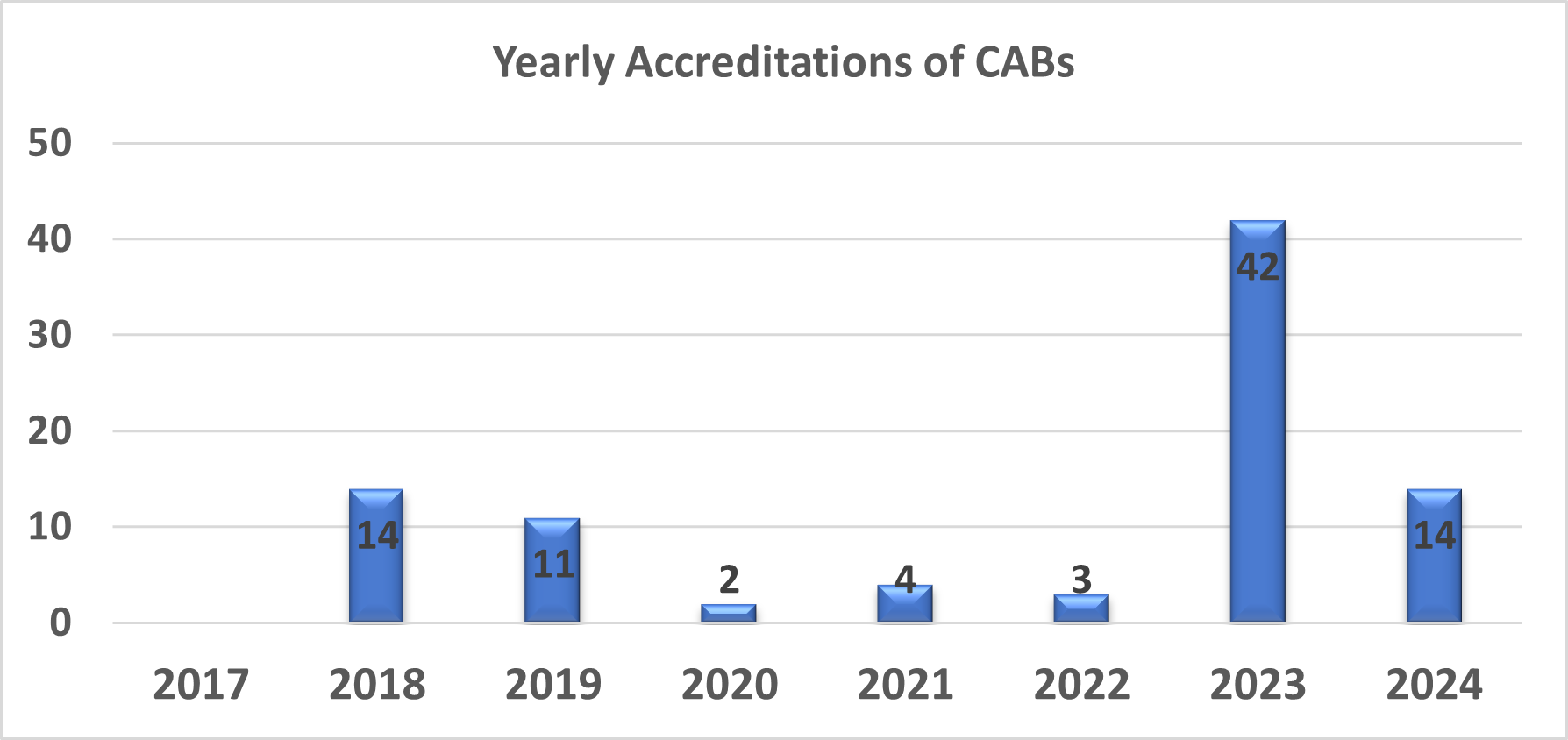
The QAI Centre for international Accreditation (QAI CIA), a fairly young organisation, started its accreditation activities for Conformity Assessment Bodies (CABs) in 2017 with the following accreditation programmes:
2017
• Accreditation of Medical Testing Laboratories as per ISO 15189
2018
• Accreditation of Testing laboratories as per ISO/ IEC 17025
2021
• Biobanking Accreditation as per ISO 20387 (For the First time in India)
2022
• Accreditation of Calibration laboratories as per ISO/ IEC 17025
2023
• Accreditation of Proficiency Testing Providers as per ISO 17043
• Accreditation of Reference Material Producers as per ISO 17034
• Accreditation of Inspection Bodies as per ISO 17020
We are happy to announce that QAI CIA achieved international recognition by joining the APAC MRA for Testing (ISO/IEC 17025) and Medical Testing (ISO 15189) on 31 October 2022 and the ILAC MRA for Testing (ISO/IEC 17025) and Medical Testing (ISO 15189) on 10 December 2022. This makes us the second AB in India to achieve membership of the ILAC MRA. We intend to add more accreditation programmes to the ILAC MRA as we progress.
This recognition has helped us in expanding and promoting our business, and accepting a greater number of applications for accreditation.
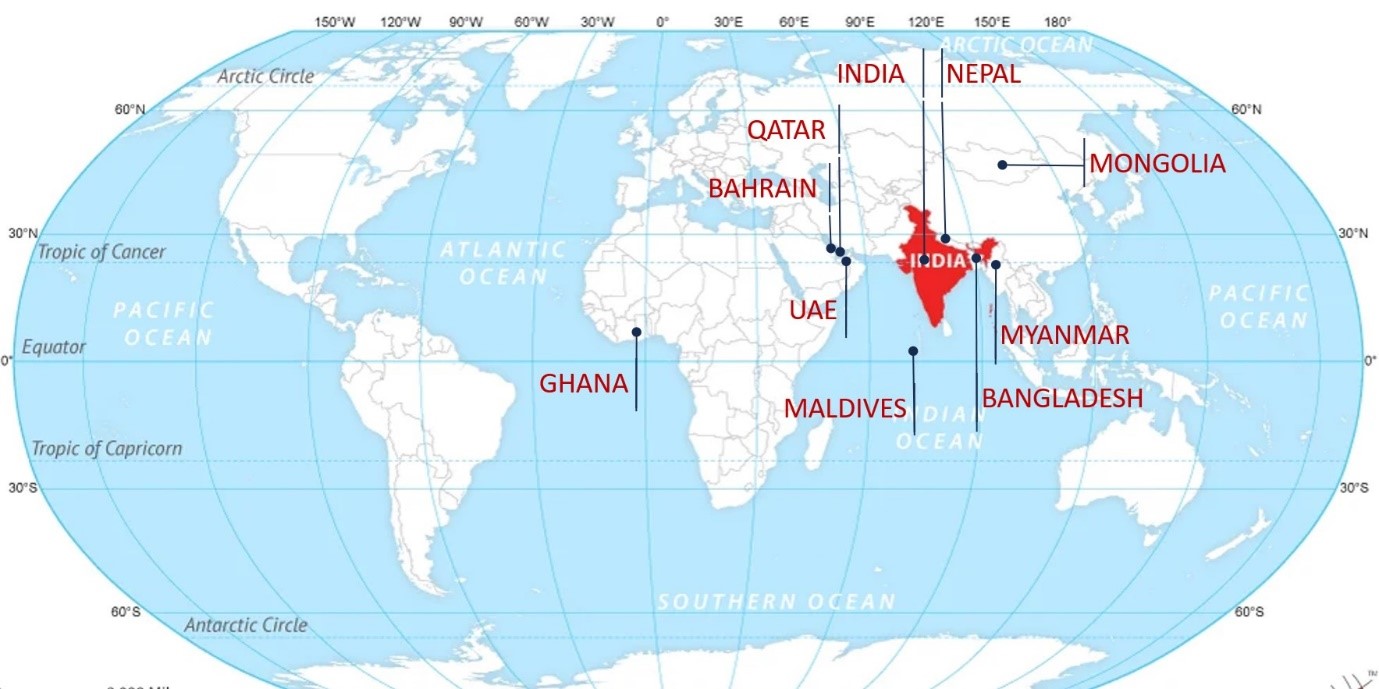
We are also pleased to announce that QAI CIA is now present in Asia, Africa and the Middle, East as an accreditation partner. We are motivated to excel in supporting countries that may not have their own Accreditation Body and / or a specific accreditation programme.
We are committed to hold to our Vision and Values by providing a credible accreditation mechanism. We are certainly would like to contribute towards our national government’s agenda of Viksit Bharat (Developed Bharat) in the conformity assessment ecosystem and do our bit to take Bharat (India) to the 1st World Ranking in Quality and Accreditation Infrastructure.
Further, we are open for bilateral collaboration to learn, share and support these shared goals with organisations in different countries.

Introduction
Natural gas is widely recognized as an eco-friendly and clean energy source, offering significant potential to address environmental concerns while meeting the increasing demand for energy sustainably. The Government of India has placed emphasis on encouraging the adoption of natural gas as both a fuel and feedstock nationwide, aiming to elevate its proportion in the primary energy mix from its current level to 15% by 2030. At present, about 23,500 Km long gas pipeline network is under operation in the country and around 12,000 KM pipeline is approved/under construction. The GoI plans to complete the vision of One Nation One Gas Grid by 2030.
Understanding Accreditation
Accreditation is the process by which an authoritative body evaluates and certifies the competence, integrity, and performance of organizations, laboratories, and individuals. First and foremost, accreditation fosters confidence among stakeholders, and also acts as a catalyst for continuous improvement. By adhering to internationally recognized standards and best practices, stakeholders are compelled to enhance their processes, technologies, and workforce competence. This not only leads to higher efficiency but also promotes innovation and sustainability in the sector.
India is among the top nations working towards enhancing the quality infrastructure within the country & beyond. India’s national accreditation system under the Quality Council of India (QCI) ranked 5th in the world in the recent Global Quality Infrastructure Index (GQII) 2021. The GQII ranks the 184 economies in the world on the basis of the quality infrastructure (QI).
The responsibility of the accreditation procedure in India rests on the shoulders of National Accreditation Board for Certification Bodies (NABCB) which is a constituent board under the Quality Council of India. It is mandated to provide accreditation to Conformity Assessment Bodies (Certification, Inspection & Validation/Verification Bodies) as per international standards, generally ISO standards for various Conformity Assessment Schemes / Standards.
CGD System across the Globe: A Comparative Study
CGD System in USA:- Approximately two-thirds of crude oil and refined products in the United States are transported through pipelines, while almost all natural gas is conveyed via the same means. Various everyday essentials such as stoves, automobiles, ovens, planes, and dryers rely on products derived from oil and natural gas. ASME B31.8-2022: Gas Transmission And Distribution Piping Systems outlines specifications aimed at ensuring the secure transportation of gas within pipeline infrastructures.
ASME B31.8-2022 addresses the entire process from design and fabrication to installation, inspection, examination, and testing of pipeline facilities utilized in gas transportation. It encompasses engineering prerequisites essential for the secure design and construction of pressure piping. Furthermore, the standard delineates safety considerations regarding the operation and maintenance of such pipeline facilities, aiming to safeguard both the general public and workers.
CGD System in the European Union:- National Energy and Utilities Regulatory Commission (NEURC) is the regulatory body for Gas distribution in the EU. Over the past few years, Ukraine has made a number of important changes in the regulation of the gas market. One of the main achievements was the adoption in 2015 of the Law of Ukraine “On the Natural Gas Market”. The new law enshrined in the EU’s economically sound approaches to the organization of the natural gas market, separate’s the functions of the operator from the functions of gas production and supply, clearly outline the functions of the state and the independence of the regulator, and establishes the principle of regulating natural monopolies and free pricing in competitive gas market segments.
Role of Inspection Bodies in ensuring Accreditation
The Inspection body accreditation is a formal means of demonstrating the technical competence to perform specific types of inspections, thereby providing a ready means for the customers to gain confidence in the quality of inspection services they will receive. Inspection aims at demonstrating the safety and functionality of the inspected target. Typical examples of inspected targets include boilers, pressure vessels, transformers, oil and gas sectors, machinery and equipment, and food processes.
The article specifically focuses on the role of Inspection Bodies in ensuring quality & competency of bodies in supply technical cluster specifically to City Gas Distribution. The City Gas Distribution (CGD) is the last component of the Natural Gas value chain delivering Natural Gas to end users in the town and cities to meet in demand for a cleaner and more efficient, economical and environment friendly energy source.
One of the primary benefits of accreditation is its role in ensuring safety. Natural gas, while a clean and efficient fuel, can pose significant risks if not handled properly. Accredited CGD operators undergo stringent assessments of their safety protocols, infrastructure, and emergency response capabilities. This results in a safer operating environment for both employees and the public, reducing the likelihood of accidents and minimizing their impact if they occur.

The Inspection Bodies seeking accreditation for Inspection comply with the requirements as specified in the international standard ISO/IEC 17020:2012 “Conformity assessment – Requirements for the operation of various types of bodies performing inspection” and other requirements. The National Accreditation Board for Certification Body (NABCB) provides accreditation to various conformity bodies in Scope sector 26 (Gas Supply as per IAF ID1:2014) that demonstrates sound capacity to conduct inspections based on three categories Type A, Type B, Type C. NABCB plays a crucial role in ensuring the safety and quality of the CGD system via accrediting various inspection bodies such as Bureau Veritas, Tata Projects Ltd., SGS India, TÜV SÜD South Asia etc. in India. The Petroleum and Natural Gas Regulatory Board (PNGRB) acts as a regulator that allows Technical Standards and Specifications including Safety Standards (T4S) audits according to Petroleum and Natural Gas Regulatory Board Act, 2006.
Challenges and Opportunities
While accreditation brings numerous benefits to the CGD sector, it also poses certain challenges. Maintaining accreditation requires ongoing investment in staff training, equipment, and quality management systems, which can be resource-intensive for smaller inspection bodies. Furthermore, keeping pace with evolving technologies, regulatory frameworks, and industry best practices necessitates continuous improvement and adaptation.
However, these challenges also present opportunities for innovation, collaboration, and knowledge sharing among stakeholders to drive excellence in CGD.
Way Forward
The accreditation of inspection bodies is crucial for elevating the quality, safety, and dependability of city gas distribution infrastructure and operations. Through adherence to globally acknowledged standards and optimal practices, accredited inspection bodies offer assurance to regulators such as PNGRB, investors, and the public that CGD networks are engineered, built, and managed to the utmost standards of excellence and safety. This endeavor not only aligns with India’s pursuit of Sustainable Development Goals 7, 11, and 13 but also reinforces the nation’s commitments to climate action as outlined in its Nationally Determined Contributions (NDCs).


Last February, the National Accreditation Body of Colombia (Organismo Nacional de Acreditación de Colombia – ONAC) embarked on a groundbreaking initiative to elevate the standards of sustainability in the tourism sector through the implementation of Colombian Technical Standards (Normas Técnicas Colombianas – NTC) in Tourism Sustainability Management Systems. This move not only signifies a strategic pivot from Sectoral Technical Standards (Normas Técnicas Sectoriales – NTS) but also underscores Colombia’s commitment to harmonizing with global benchmarks, thereby enhancing the quality and sustainability of its tourism industry.
The new sustainable tourism accreditation service was presented to the Colombian Association of Travel and Tourism Agencies (ANATO), some tourism service providers, and other stakeholders. The event served as a meeting between different stakeholders in the tourism sector, among them the Conformity Assessment Bodies (CABs), one of the key stakeholders and the ones directly interested in acquiring sustainable tourism accreditation services.
Additionally, the event served to raise awareness of the importance of accreditation, particularly in the tourism sector. Accreditation has the potential to contribute to the internationalization of tourism services provided within the country and to make Colombian tourism service providers known to a global audience.


Tourism, a rapidly growing industry, holds the promise of economic prosperity, employment generation, and the promotion of cultural and environmental awareness. However, its unchecked expansion can lead to adverse impacts, including cultural dilution, environmental degradation, and the depletion of local resources. Recognizing these dual facets of tourism, the World Tourism Organization has defined sustainable tourism as a model that fully considers its current and future economic, social, and environmental impacts, addressing the needs of visitors, the industry, the environment, and host communities.
This model aligns seamlessly with the Sustainable Development Goals (SDGs), particularly targeting the promotion of sustainable tourism that fosters job creation and local culture and products. In this light, the Colombian Ministry of Commerce, Industry, and Tourism (MinCIT), in collaboration with the Colombian Institute for Technical Standards (ICONTEC), has spearheaded the transition to NTC in tourism sustainability management, heralding a new era of standardized, high-quality, and sustainable tourism practices.
The transition from NTS to NTC reflects a profound evolution towards a more integrated and formalized framework of standardization within Colombia, setting a new benchmark for quality and sustainability in tourism at a national level. This evolution is a testament to Colombia’s deep-seated commitment to excellence and responsibility in its tourism sector. By aligning with NTC, service providers are not only adhering to national standards but are also positioning themselves competitively in the global market, enhancing their appeal to a more sustainability-conscious clientele.
The significance of this shift cannot be overstated, as it paves the way for a more resilient and competitive tourism industry that is in harmony with environmental conservation, cultural respect, and social responsibility. Moreover, the certification under NTC for sustainability in tourism acts as a catalyst for innovation, fostering economic resilience, attracting investment, and raising awareness about sustainable practices among both providers and tourists alike.
Despite the promising outlook, the heterogeneity of Tourism Service Providers (PSTs) and the financial burden of certification pose considerable challenges. The ONAC’s market study reveals a pressing need for collaborative efforts between government entities and accreditation body to facilitate certification for PSTs, overcoming financial and technical barriers. It suggests tailored incentives, tiered fee structures, and comprehensive support programs to democratize access to certification, ensuring that all segments of the tourism industry can embark on the journey towards sustainability.
This study was prepared by the Coordination of Socioeconomic Studies for Quality Infrastructure, a new area of ONAC in charge of conducting economic research and generating valuable information to support decision-making by ONAC, the CABs, the other actors of the quality infrastructure, as well as other stakeholders. The study can be consulted both in Spanish and English in the following website: https://onac.org.co/en/market-study-of-accreditation-services-in-colombian-technical-standards-for-tourism-sustainability-management-systems/onacs-blog/
There have been several studies conducted by the area, including market analysis studies for the opening of new accreditation services, review of sources that allow understanding the current state and opportunities for improvement of the quality infrastructure in Colombia, reviews of the impact of accreditation on aspects such as energy efficiency or international trade, among other documents. All these studies will be available for public consultation through ONAC’s website.
ONAC’s initiative to offer accreditation services in NTC for Tourism Sustainability Management Systems represents a significant leap towards aligning Colombia’s tourism sector with global sustainability standards. This endeavor not only enhances the competitiveness and attractiveness of Colombia as a sustainable tourism destination but also reaffirms the country’s commitment to responsible tourism practices that safeguard its cultural and natural heritage for future generations. The journey ahead is challenging, yet with strategic partnerships and sustained efforts, Colombia is poised to become a beacon of sustainable tourism on the global stage.

Italy, like most countries, is facing increasingly grave environmental and climate crises, which can affect the availability of potable water. There are also issues with partly obsolete water infrastructure, which lead to leaks and dispersion. The availability and sustainable management of water and of health and hygiene structures, have become one of the 17 objectives of the UN 2030 Agenda for sustainable development, and the European legislator intervened on the topic with the EU Directive 2020/2184. The EU Directive was implemented in Italy in March 2023, through Legislative Decree 18/2023 (the Decree).
The Decree was developed thanks to an extensive partnership between institutions, public and private stakeholders and Accredia, the Italian Accreditation Body. Federico Pecoraro, Deputy Director of Accredia’s Testing Laboratories Department, underlined the important role of tests carried out under accreditation: “In this area, accreditation continues to be a valid tool used by the authorities to establish the level of competence, impartiality and good organizational functioning of those who must ensure a high level of protection of public interests.”
Italian law and EU regulation
Water intended for human consumption can be processed or non-processed water used for drinking, preparing food and drinks or other uses, supplied through a distribution network or in tanks, bottles or containers. This also includes water used for food production and other substances for human consumption, but natural mineral waters are excluded. With the aim of ensuring healthy and clean water, Legislative Decree 18/2023 introduced important innovations for operators of potable water systems, for people involved in water treatment, and for citizens. Accreditation was already mandatory in line with the previous Decree of the Ministry of Health of 14 June 2017, but with the Legislative Decree of 2023 it has become central.
In this way, the role of accreditation for testing laboratories according to the standard ISO/IEC 17025 “General requirements for the competence of testing and calibration laboratories” was strengthened in the areas involving accredited tests on water and the certification of materials in contact with water, as well as management systems of potable water companies and the inspection of distribution networks. Article 6 of the Decree – “General obligations for a risk-based approach to water safety” explains how this approach is aimed at covering “the entire potable water supply chain, including compliance with the specifications in article 5″, and to guarantee “the continuous exchange of information between the operators of potable water distribution systems and the competent authorities in health and environmental matters.”
Accreditation of tests conducted on water
Accreditation of tests on tap water guarantees its healthiness and increases the competences of those who have a key role in the protection, control and monitoring of water resources and their natural environments. Accredited tests guarantee the safety and effectiveness of periodic monitoring of water quality by water service operators, i.e. anyone who supplies water intended for human consumption to third parties.
Out of a total of 1,353 testing laboratories accredited by Accredia (as of 31 December 2023), approximately 60% also conduct tests on water for human consumption. The Decree defined a new list of parameter values to evaluate the quality of water, determined by means of tests carried out by laboratories accredited by Accredia according to ISO/IEC 17025.
Accreditation to certify chemical reagents
The Decree also introduced important innovations regarding the so-called ReMaf, i.e. the chemical reagents and active and passive filtering materials to be used in the processing of water for human consumption. By 12 January 2036 the ReMaf will have to be certified under accreditation and subsequently authorized by the National Center for Water Safety (CeNSiA) and registered in the AnTeA system, in order to be placed on the market.
From a technical point of view, ReMaF are certified by accredited bodies that perform periodic inspections of the production plants, taking samples to be analysed and, for the analyses necessary for certification, they rely on accredited testing laboratories.

Accredited testing laboratories for the construction and civil works sector in Colombia.
By: Paola Aguirre V., Diana Jácome M., and Mauricio Rodríguez R. International Technical Direction. National Accreditation Body of Colombia – ONAC
Let’s start with a little history about accredited laboratories in Colombia.
When we talk about accredited laboratories, it is important to highlight the fact that the first accreditation granted by ONAC in laboratories scheme, was to a construction and civil works one. It was in 2009 (14 years ago), the same year ONAC started with the accreditation activity in the country.
Today, this is the sector with the highest number of accredited testing laboratories (52) that represents 19% of the total number of accredited testing laboratories. Thus, since 2019 a growth trend has been evidenced in this sector of laboratories, as identified in Graph 1, the highest growth rate was achieved in 2021, reaching 10 new accredited laboratories. Moreover, the first semester of 2023, accreditation has been granted to 3 new laboratories and there are another 5 in the process of accreditation, maintaining the growth trend that started since 2019.
In addition, it is worth mentioning that since 2014, the testing laboratories scheme has the international recognition signed with the InterAmerican Accreditation Cooperation (IAAC) and the International Laboratory Accreditation Cooperation (ILAC), which are multilateral agreements that allows around 100 economies worldwide to accept and recognize the results issued by testing laboratories accredited by ONAC.
Graph 1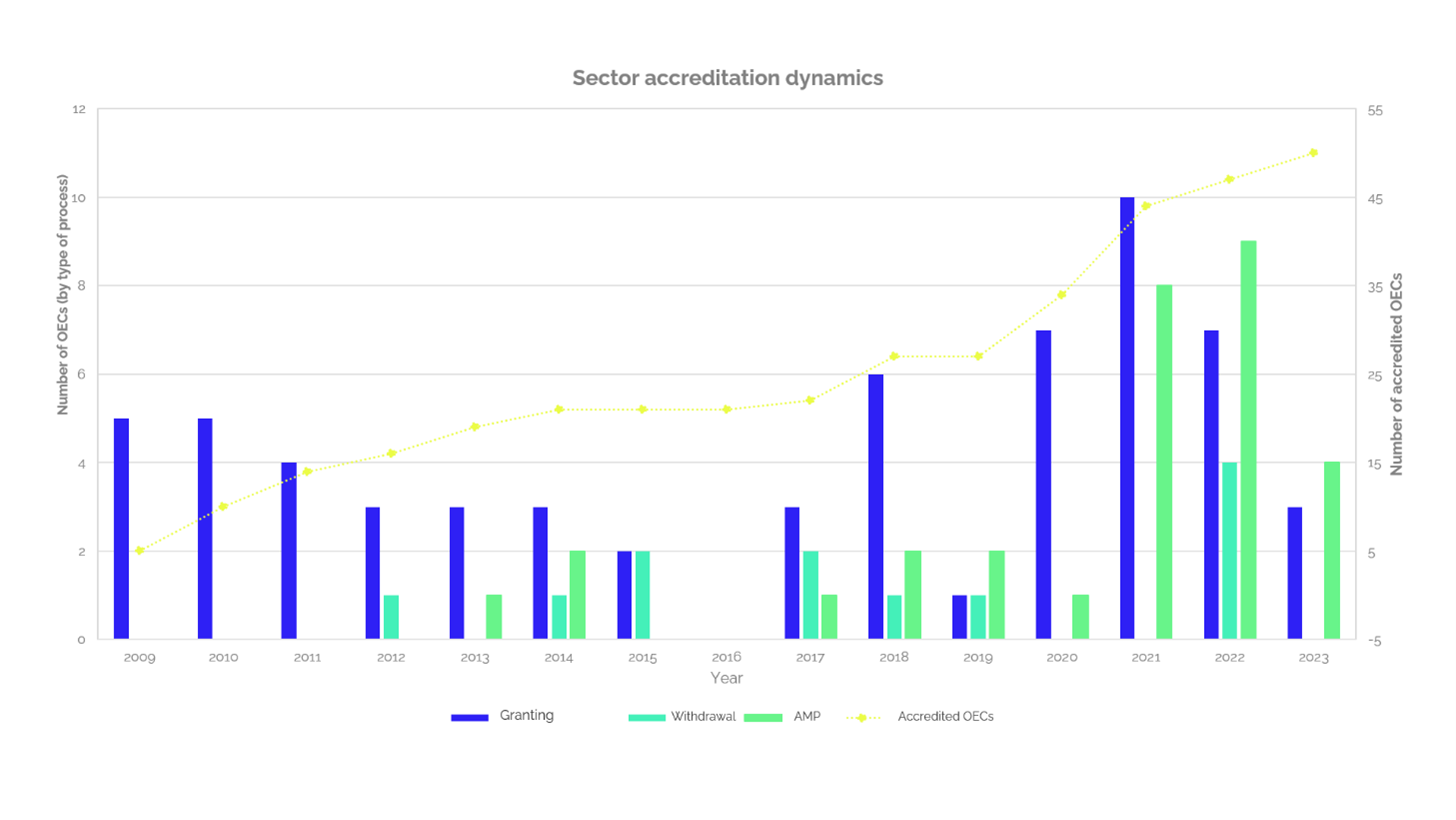
Additionally, Graph 1 also shows the number of new laboratories accredited pr year (grants), the increase in the number of testing or the number of sites (expansion), laboratories that have had their accreditation withdrawn (withdrawals), and the net number of accredited laboratories in the sector. Thus, since the first accredited laboratory, the sector achieves an average growth rate of 3.3 new laboratories per year.
Regarding the withdrawals of accreditation, no clear trend is observed, these are considered isolated cases, with a maximum of 4 for 2022, 2 in the years 2015 and 2017, and values of 1 or 0, for the other years. On the other hand, in the last years there is also an increasing trend regarding the expansion of accreditation, taking into account that only until 2013 the first expansion of the scope was presented by one laboratory accredited in the construction sector, with values between 1 or 2 laboratories that expanded their accredited scope between 2013 and 2020.
In the last two years there was a substantial increase in applications, 8 laboratories in 2021, 9 laboratories in 2022 and 4 so far in 2023 have expanded their accredited scope, showing that not only in the last two years the number of accredited laboratories in the sector has increased, but that laboratories are seeking to expand the portfolio or capacity of their accredited services to meet market needs.
The fact that the number of accreditations for testing laboratories in this sector is predominant with respect to the other sectors of laboratories accredited by ONAC, demonstrates the importance and confidence that the user of these services, recognize in accreditation, which is a fundamental element to confirm the competence to guarantee the validity of the results that are issued, from which relevant decisions will be taken within the processes of planning and execution of the constructions and civil works.
Overview of accredited laboratories
When we talk about accredited laboratories, it is important to keep in mind which is the scope of each accreditation, i.e., which are the specific activities that each laboratory performs competently. These scopes are dynamic and depend on the services that each laboratory has demonstrated that can perform competently, including, in effect, the traceability of its measurements to the International System of Measurements (SI).
One of the aspects that are part of the accredited scope are the laboratory locations, from which three types of laboratories can be differentiated: permanent, on-site and mobile laboratories. The first ones, are those that perform the tests in the permanent location of the laboratory, which is clearly registered in the accredited scope and using equipment that cannot be moved from one location to another.
The second category is characterized because the tests are performed with portable equipment, directly at the sampling site, which may be at the front of the construction or civil works, delivering results immediately in most cases. And the last one, it is the complete laboratory that moves to the sampling site, regardless of the nature of the equipment used in the tests. Each type of laboratory presents a series of conditions, restrictions and benefits when providing services, but, as long as they are covered by the accreditation granted by ONAC, they comply with the elements of competence established in the international standard ISO/IEC 17025 and the requirements of the International Laboratory Accreditation Cooperation (ILAC).
Currently, ONAC has 52 accredited Conformity Assessment Bodies (CABs) for this sector, legal entities that subscribe the accreditation with ONAC. The operational headquarters or Laboratories reach a total of 67 (permanent site) covering 11 departments from 32 in whole country, and 20 cities and/or municipalities, information that can be identified in Figure 1. Additionally, fourteen (14) CABs have laboratories that are accredited to perform on-site testing and one (1) corresponds to a mobile laboratory, being this the only sector that has this type of laboratory.
Graph 2
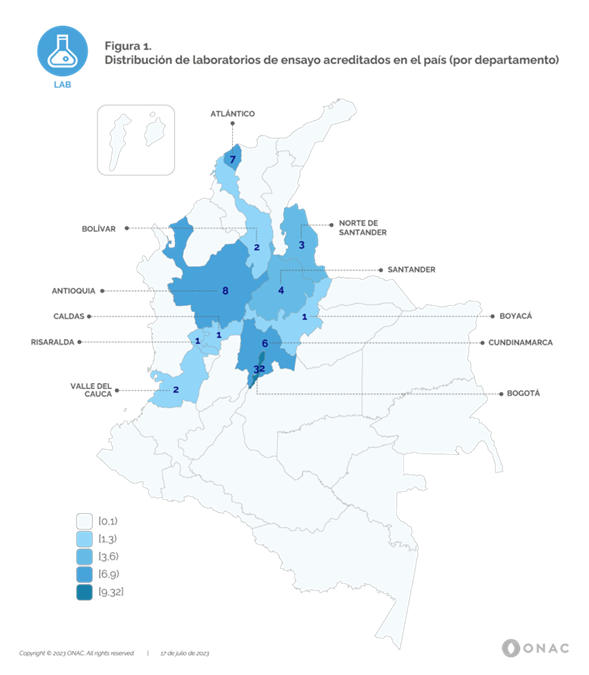
As can be seen in Figure 1, the largest number of accredited laboratories are located in the city of Bogotá, D.C., with 32 laboratories, equivalent to 48% of the total, Antioquia has 8 laboratories, representing 12%, Atlántico with 7 laboratories, reaches 10%, Cundinamarca with 6 laboratories equals 9%, Santander with 4 laboratories accredited laboratories reaches 6%, Norte de Santander with 3 laboratories reaches 5%, Valle del Cauca and Bolívar with 2 laboratories, 3% each one and Risaralda, Caldas and Boyacá with 1 laboratory, correspond to 1.5% each one. In general terms, testing activities for the sector are centralized in the departments of the Andean, Caribbean and Pacific regions, covering the main capital cities. It is important to clarify that the 14 on-site laboratories and the mobile laboratory cover the entire national territory.
When we refer to the scopes accredited in Construction laboratories, it is possible to analyze them from several aspects, starting with the type of testing provided, as shown in Graph 2, in which tests under mechanical techniques predominate, reaching 40% of the total accredited tests offered by the accredited laboratories, tests that include: tensile, tension, breakage, compression, among others, to determine material resistance.
Graph 3
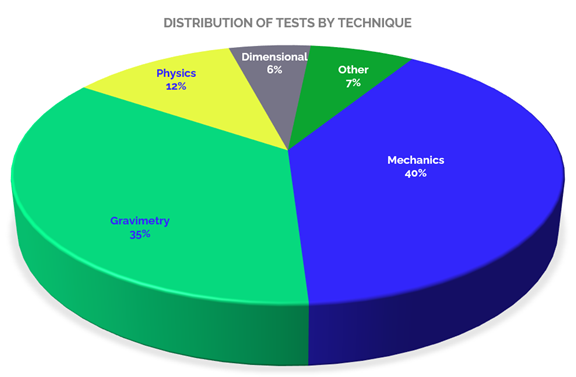
Secondly, there are gravimetric techniques, which constitute 35 % of the total number of accredited tests, including: moisture determination and ratios, particle size distribution, aggregate index, density, among others. 12% corresponds to physical tests, which include the determination of surface quality, linear mass, moisture content by distillation, among others. 6 % of the accredited tests are dimensional, with tests such as: measurement of projections in bars, thickness of compacted specimens of asphalt mixtures, measurement of graphite and wires, among others.
And finally, the remaining 7% (Others) corresponds to tests performed under various techniques such as: physicochemical, colorimetry, optical emission spectrophotometry, rheology, among others. All these tests are of great relevance for the sector, as they allow to determine the quality of materials and items, and thus guarantee their reliability and adequacy in the construction processes.
Another way to classify the accredited scopes of the sector’s laboratories corresponds to the test item, although a laboratory may have within its portfolio, for example, tests in the mechanical field, this is limited to a detailed group of items, for which it has demonstrated that it can provide services competently. Graph 3 shows the distribution of accredited tests by type of item.
Graph 4
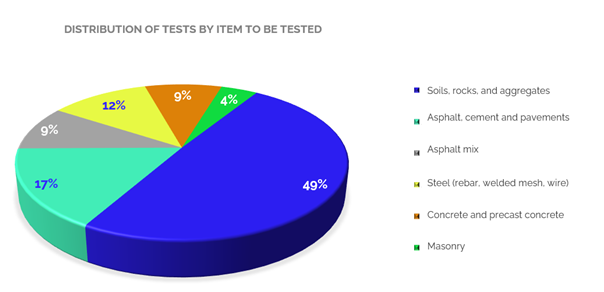
The accredited tests cover several types of items and materials. Among these, we find tests for soils, rocks and aggregates (fine and coarse aggregates), which are mostly gravimetric and the most offered in the sector, reaching 49 % of the total accredited scopes. Then, with 17 % are the tests performed on asphalt, cement and pavements, including hydraulic cement, and with 9 % the tests on asphalt mixtures, in which for these two types of materials the tests are predominantly of mechanical and gravimetric type.
Accredited tests on steel materials (rebar, smooth bars, electro welded mesh, smooth and drawn steel wire, among others) represent 12 % of the total number of tests, and for this type of materials, most of the tests are mechanical. Tests on concrete (including hydraulic concrete) and prefabricated concrete, in which mechanical compression and flexural tests predominate, represent 9 %, and tests on masonry materials (paving blocks, slabs, bricks, blocks, etc.) of mechanical type represent 4 %.
Although the materials and items mentioned above are those that predominate in the construction and civil works sector, there are also accredited testing laboratories that offer services for complementary materials and items or items of indirect use for the sector, such as electrical elements, plastics, pipes, among others. All these scopes and the accredited laboratories that provide these services can be consulted in the Official Directory of Accredited Laboratories – DOA.
If we analyze the accredited scopes by type of standard or testing standards used for the provision of services, we can identify a distribution as shown in Graph 4. In which we can see that 47% of the tests offered by accredited laboratories in this sector are performed according to the guidelines of the standards of the National Roads Institute (INVIAS), which is probably related to the needs generated by the institutions that outline the requirements in the contracting of public civil works.
Graph 5
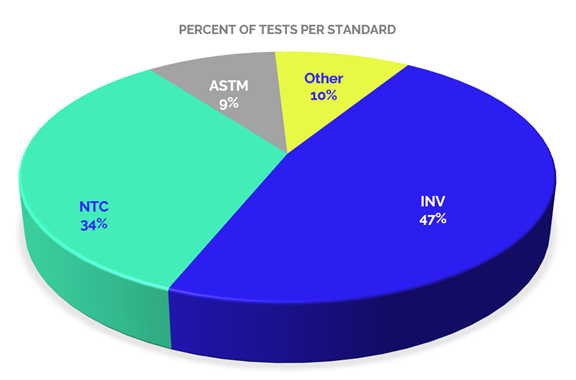
While 34 % of the accredited tests are performed under National Technic Colombian standard – NTC standards, issued by ICONTEC, recognized by the Colombian Government as the National Standardization Organization, these standards are usually adoptions of international standards or standards developed by national standardization committees. In a low percentage, 9%, there are tests with ASTM international standards, and 10% with other standards such as AASHTO (American Association of State Highway and Transportation Officials), API (American Petroleum Institute), among others.
Considering that almost half of the tests are performed with INVIAS standards, documents based on NTC standards, which are derived from international standards such as ASTM, it is necessary to call attention to the fact that these standards are outdated in relation to the international documents on which they are based (mainly 2013).
This is due to the fact that the speed with which international standards are produced and updated is greater than the speed with which these documents are adopted in Colombia; even more so, when they are included in National Technical Regulations or in the terms for contracting of these services by the State. This lack of updating represents a competitive disadvantage for national laboratories in relation of the mutual recognition agreements that ONAC has signed with ILAC.
Finally, from the information available in ONAC, it is possible to identify the type of organization that owns the accredited laboratories. Most of the accredited laboratories in the sector correspond to private entities, reaching 85%, being clear that it is this type of entities that can adjust their strategies, budgets and operations in a more dynamic way to the market demand in this economic sector. The remaining 15% of the laboratories correspond to public entities. Now, considering the importance of this sector in a developing country like ours, it is highlighted that the academy has taken part in this sector, 9 laboratories are identified ascribed to educational institutions, reaching a participation of 17 %, three laboratories of SENA (National Learning Service) and 6 laboratories of universities, of which two correspond to public universities.
Some reflections
The analysis carried out allows identifying the growth trend of accredited testing laboratories for the construction and civil works sector, as well as some classifications that can be made based on the accredited scopes and the legal nature of the accredited laboratories, but it also allows showing the areas, specific sectors, techniques, items or materials, where there is no supply or the existing supply is insufficient.
An economic and growth sector, such as construction and civil works, in terms of infrastructure for a country owes its success, growth and contribution to society, in part to the quality assurance of its processes, as well as the search for efficiency in its construction methods, for which accredited conformity assessment services, such as the activities performed by testing laboratories, are fundamental and indispensable, in the different stages, from planning, to the confirmation of designs and compliance with specifications once the works or constructions are completed.
For this reason, having a greater capacity of accredited testing, with a greater offer and with the globally accepted level of competence, is a strategic aspect in the competitiveness of the sector.

2. Conferences

The inspection division of Cofrac in now offering a new accreditation scheme for the control and verification of removable structures to ensure the safety of people.
What is a removable structure?
A removable structure is a temporary and dismountable structure for sporting, cultural, commercial, or tourist events and consists of a framework that can be repeatedly assembled and dismantled for temporary use.
Removable structures are divided in two groups:
Context and challenges of this accreditation scheme
In France, until 2022, there were no rules for verifying the mechanical strength of demountable structures, or for assessing an organization’s ability to control and verify them. However, the industry had created a practical guide that the safety commissions also relied on.
With the Paris 2024 Olympics Games approaching and safety issues at meetings associated with major gatherings, it is necessary to fill the regulatory gap.
A working group has been set up by the French Ministry of the Interior and Overseas Territories to draw up a technical reference system setting out the design, installation and operating rules, as well as the inspection and verification methods for these structures. This work was based on the “Practical Guide
– Dismantable Equipment and Assemblies” drawn up in 2017 by SYNAPSE and the “Good Practice Guide
– Dismantable Equipment and Assemblies” of the Paris Police Prefecture.
Cofrac was also involved in this work due to the Ministry’s decision to rely on accredited organizations to carry out these inspections.
What are these checks and inspections?
Controls and checks are divided into two phases:
On October 1, 2022, Cofrac launched the accreditation scheme according to the NF EN ISO/IEC 17020 standard for two areas of inspection: the design control and the verification of assembly and operational inspection. The assessment plan relies on the decree published on August 5, 2022, setting out the safety rules, technical provisions, inspection control obligations and conditions applying to these temporary and dismountable structures.
This scheme has two specificities:
Accreditation is mandatory for organizations that are not approved technical inspectors. It therefore constitutes a real opportunity for these organizations to be able to provide this type of service, in view of their broader markets and skills.
Accreditation already existed for similar activities such as scaffolding, mechanical shelving and structural strength testing. To meet the demand for accreditation, Cofrac extended the qualifications of some of these technical assessors to these new schemes and has also qualified new assessors.
To date, 4 organizations are accredited for these new schemes, and others are in the process of becoming accredited.
Accreditation will be mandatory from 1 January 2024 for all inspection bodies wishing to operate in this technical field.

EIAC celebrated World Accreditation Day
The Emirates International Accreditation Centre (EIAC) celebrated world accreditation day. In line with this year’s theme “Supporting the Future of Global Trade” EIAC arranged various activities with the collaboration of stakeholders and regulatory authorities. The main event was held in the JW Marriot Marquis hotel in Dubai. The event was attended by representatives from accredited conformity assessment bodies, Ministry of Industry and Advanced Technology, UAE, Dubai Health Authority, Food Safety Department and industry. Addressing the audience, Ms. Amina Ahmed Mohammed, the Chief Executive Officer of the Emirates International Accreditation Centre (EIAC), highlighted the importance of accreditation and the role the International Laboratory Accreditation Cooperation (ILAC) and International Accreditation Forum (IAF) play in supporting global trade. She said the future of global trade is expected to be technology driven where globally recognized standards will have utmost importance. Dr. Farah Al Zarooni, Assistant Undersecretary of UAE Ministry of Industry and Advanced Technology, addressed the gathering saying UAE in general and Dubai specifically, have become a global hub for trading. She highlighted the various government initiatives in supporting global trade through developing standardized and efficient inspection methods and adopting technological advancement in ports and shipping operations. A panel discussion titled “from local to global” was also part of the event.

EIAC CEO Ms. Amina Ahmed Mohammed addressing the World Accreditation Day celebrations
Safety of Fairground & Amusement Equipment is paramount
In 2022, the UAE has seen significant growth in the travel and tourism sector that has contributed to 9% of the total GDP, while Dubai alone has seen a growth of 53.8% compared to 2021. According to “Euromonitor’s Top 10 City Destinations Index 2022”, Dubai was ranked the second most visited city in the world.
Addressing a tourism seminar in Dubai, Ms. Amina Ahmed Mohammed, the Chief Executive Officer of the Emirates International Accreditation Centre (EIAC), said Dubai is a popular destination for amusement parks equipped with advanced indoor and outdoor amusement equipment, serving millions of visitors yearly. Other Emirates of the United Arab Emirates (UAE) including Abu Dhabi are also home of various branded theme parks. Thus, safety of fairground and amusement equipment used in these parks is paramount. She said the EIAC’s accreditation schemes for inspection bodies in Fairground and Amusement Equipment provides assurances to the public about the safety of amusement rides and play areas. Ms. Amina Ahmed Mohammed said EIAC also has an accreditation scheme for the certification of persons who are working in the amusement parks and fairgrounds, that further enhances the prospects of safe operations in the sector. It was further noted that the EIAC’s accreditations are globally recognized.
The leisure and entertainment sector plays a vital role in achieving the ‘UAE Tourism Strategy 2031’ that aims to significantly increase the tourism sector’s contribution to the nation’s economy.
Addressing the seminar, Engr. Yousef Ahmed Aljasmi, the Director of Inspection Bodies Accreditation Department of EIAC, provided input on EIAC’s plans in reinforcing the quality infrastructure of the Leisure and Entertainment sector by continually supporting the development of the local Inspection and testing bodies in collaboration with regulators and stakeholders. Engr. Yousef said the EIAC’s accreditation scheme covers the design review, manufacturing process, initial and in-service examinations and inspections.

Engineer Yousef Ahmed Al Jasmi addressing the seminar
EIAC’s unique and new accreditation scheme for certification bodies
The United Arab Emirates (UAE) is the home of people from more than 200 nationalities. The concepts of tolerance and coexistence in the UAE are considered fundamental in maintaining social harmony and peace based on cultural pluralism, accepting others, and rejecting discrimination, hatred, and intolerance in society. The UAE has issued UAE Standard UAE.S 5037: 2021 “Tolerance and Coexistence” for promotion of tolerance and coexistence.
The Emirates International Accreditation Centre (EIAC) has launched a new accreditation scheme for management systems certification bodies according to ISO 17021-1 main accreditation criteria for UAE S. 5037: 2021 “Tolerance and Coexistence management systems.
The Emirates International Accreditation Centre (EIAC) is also working to launch a new accreditation scheme for persons certification bodies in collaboration with Health and Safety Department of Dubai Municipality. The main accreditation criteria is ISO 17024 and the scheme is for the certification of “Health and Safety officers for labor accommodations”.
EIAC attended IAF-ILAC and ARAC mid-term meetings
Delegations of Emirates International Accreditation Centre (EIAC) attended the mid-term meetings of IAF-ILAC and ARAC. THE IAF-ILAC mid-term meetings were held in Belfast, Northern Ireland and the ARAC mid-term meetings were held in Manama, Bahrain. Ms. Amina Ahmed Mohammed, CEO of EIAC, and the current chair of ARAC lead the EIAC delegations. Delegates from member bodies attended the meetings.

EIAC’s delegation attended IAF-ILAC and ARAC mid-term meetings
EIAC conducted various trainings for laboratories and inspection bodies
The Emirates International Accreditation Centre (EIAC) has conducted various trainings for laboratories and inspection bodies in specialized sectors.
A special workshop for the accredited Inspection Bodies in accordance with ISO/IEC 17020 & ILAC P15 was conducted. The target audience of the workshop was the inspection bodies that are working in the field of inspections of Fairground and Amusement Equipment for the scope of independent (in-service) examination. The aim of this workshop was to enrich the market with locally based Inspection Bodies to sustain the continuity of businesses managing a variety of complex attractions in Dubai, Abu Dhabi and other emirates.
ISO/IEC 17025 training for experts of the Federal Authority for Nuclear Regulation (FANR) was conducted in Abu Dhabi in June. Mr. Ahmed Saad Was the resource person.

EIAC’s training for inspection bodies for amusement parks and fairgrounds inspection

EIAC’s training on ISO/IEC 17025

EIAC’s training on ISO/IEC 17020
Training on proficiency testing was conducted in July in Dubai with Mr. Mohammad Saaed as the resource person.
Training for medical laboratories was conducted in September and Dr. Venkatesh Thuppil was the main resource person.

Municipal pipelines such as water supply and drainage, electricity, gas and communication are buried under urban roads which are affected by vehicle load, vibration, groundwater level and other factors. Disasters such as pavement collapse and cracking, are easily caused which result in inconvenience to urban production and life and may even cause personal injury. This is particularly the case in recent years, with the further expansion of the urban scale and the increasing density of underground pipe network in some cities. Geological disasters of urban roads are occurring frequently, which has attracted the increased attention of the municipal departments and the general public.
Similarly, the application of robot technology in the inspection of internal defects of urban sewer and non-excavation pipeline repair technology has attracted wide attention because of the advantages of high repair efficiency and low repair cost.
After a period of service, the materials used in urban roads and urban sewers gradually age and their functions decline potentially resulting in geological disasters and potential safety hazards. In order to find the potential safety hazards and take measures to eliminate them, it is necessary to regularly evaluate the risk of urban road underground failures and the internal defects of urban sewer. Since accreditation is an affirmation of the management and technical competence of the inspection body, there is an increasing demand for accreditation in the competence of risk evaluation in underground failures related to urban roads and the competence in internal defect evaluation of urban sewers.
CNAS has actively responded to the accreditation needs in the above fields organizing experts to study and discuss the characteristics and technical requirements of inspection and testing activities in these two fields, and promote accreditation of the inspection bodies. Following analysis, the inspection items for urban roads is the detection and evaluation of attributable characteristics of underground failures such as cavities underneath the pavement, voids, loosely infilled voids, water-rich voids in the area using detection methods such as ground penetrating radar method for urban roads according to relevant technical standards, and to carry out a risk evaluation. The inspection items for urban sewers are sewer defects and condition evaluation, including the detection of structural and functional defects of urban sewers using closed circuit television inspection and quick view pipe inspection methods using the relevant technical standards Then to evaluate and judge the structural and functional conditions of the sewer and calculate the rehabilitation index and maintenance index. These two types of activities include the typical characteristics of inspection activities. Therefore, inspection accreditation in accordance with ISO/IEC 17020 is applicable.
CNAS is accrediting inspection bodies in these two technical fields. Competence in the detection and evaluation of underground failures in urban roads and sewer defect and condition evaluation of urban sewers is accredited according to the inspection body accreditation standard ISO/IEC 17020. As of August 2023, in the above fields, a total of 31 inspection bodies have been accredited by CNAS, with more inspection bodies currently in the process of seeking accreditation for these activities.

Reference laboratories are not referred to in the context of accreditation as often as calibration or testing laboratories. However, during COVID-19 epidemic, it was reference laboratories that came into the focus of increased public attention due to stories about the possibility of their production of dangerous bacteria and viruses, which at some point, intentionally or accidentally, can break out of the laboratory walls. This reputational risk was unexpectedly raised in relation to the reference laboratories of Kazakhstan potential involvement in the development of biological weapons. As a full member of ILAC, the National Center of Accreditation (NCA) of the Republic of Kazakhstan would like to tell you about how the activities of reference laboratories are regulated in our country.
Kazakhstan is an exceptionally peaceful country, where more than 100 nationalities live in harmony on a large territory. Commitment to everything that contributes to the continuation of this peaceful life is not just a national ideology – it is woven into the consciousness and subconsciousness of Kazakhstanis. Therefore, any accusations of unseemly actions against human civilization are perceived very painfully in our country.
The history of 2020 ended well when diplomats and the scientific community of our country explained to the public how and what reference laboratories actually work on, and that their activities are kept in strict accordance with the scope of accreditation, that is, exclusively for medical and civil purposes.
There are seven reference laboratories in Kazakhstan. Their listing is approved by the order of the Minister of Health of Kazakhstan, and it is impossible to get into this list without permission and control.
In the Council of Europe, reference laboratories are structural divisions of the Ministry of Health of Kazakhstan, and the tasks that they solve are carried out under the control of the Ministry.
In the Council of Europe, reference laboratories of Kazakhstan are accredited entities under the national standard of ST RK ISO 15195-2018 “Laboratory medicine. Requirements for reference measurement laboratories”, identical to the international standard ISO 15195:2018 Laboratory medicine – Requirements for reference measurement laboratories. Of course, this is not a new standard for the world of standardization, but given 30-years of experience as an independent state, Kazakhstan sees it as a good tool for improving the country’s healthcare system.
Thus, as an accreditation center, we would like to once again remind you of the importance of reference laboratories that focus on studying microorganisms to protect humanity from possible harm.