 |
|
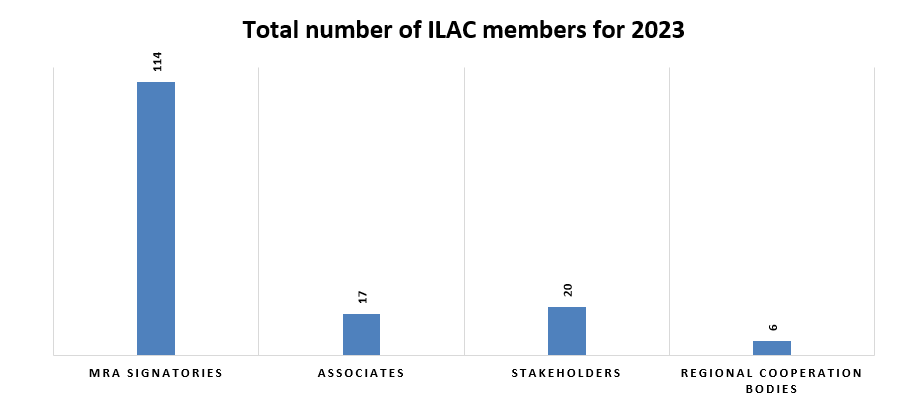
ILAC’s international network of members consists of organisations from a diversity of global economies. These members include signatories to the ILAC Mutual Recognition Arrangement (MRA), Associates, Stakeholders and Regional Cooperation Bodies. |
 |
|
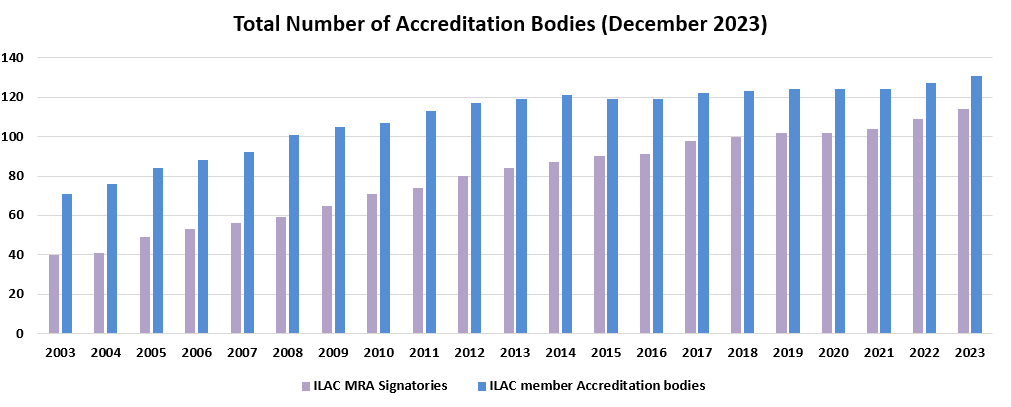
Since the formation of the ILAC MRA in 2000, the number of accreditation body members from around the world has more than doubled. These accreditation body members comprise the ILAC MRA Signatories (Full Members), as well as Associate members who are working towards achieving their ILAC MRA signatory status. |
 |
|
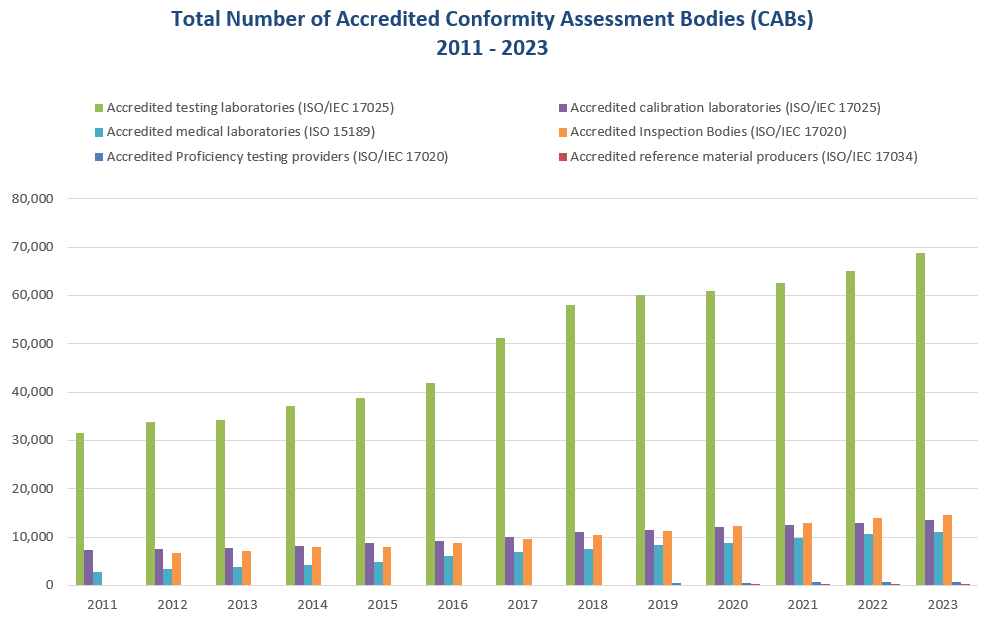
2023 Over 93,279 laboratories, almost 14,549 inspection bodies, over 746 proficiency testing providers and over 308 reference material producers were accredited by the ILAC MRA Signatories in 2023. 2021 In November 2021, the ILAC MRA was extended to include the accreditation of biobanking as a Level 2 activity, to the criteria specified in the Level 3 normative document: ISO 20387 Biobanking – General requirements for biobanking as a standalone standard. 2020 2019 2012 2001 |
 |
|
The current scope of the ILAC MRA includes the accreditation of calibration laboratories using ISO/IEC 17025, testing laboratories using ISO/IEC 17025, medical testing facilities using ISO 15189, inspection bodies using ISO/IEC 17020, proficiency testing providers using ISO/IEC 17043 and reference material producers using ISO 17034. |