
The Czech Accreditation Institute (CAI) was established as a national accreditation body by the Czech Republic on 1st January 1993. In January 2023, a festive meeting of CAI staff was held to celebrate the 30th anniversary. The meeting took place in the splendid surroundings of the historic town of Jindřichův Hradec, founded by the royal family of the Přemyslid in the 10th century.

Participants recalled the history of the establishment and operation of the CAI from its earliest beginnings to the present day.
In 1996 the initial peer-evaluation of CAI was undertaken and in 1998 the CAI signed the EA Multilateral Agreement (MLA) to become the first EA MLA signatory in Central and Eastern Europe and in doing so gained international recognition.
Read More
The OIML and the BIPM are pleased to announce that the 2023 World
Metrology Day Resource Website is now live:
https://www.worldmetrologyday.org/
The theme in 2023 is “Measurements supporting the global food system”.
This theme was chosen because of the increasing challenges of climate
change, and global distribution of food in a world whose population
reached 8 billion at the end of 2022.
The 2023 poster was designed in association with Sistema Interamericano
de Metrología (SIM) and the Instituto Nacional de Tecnología Industrial
(INTI), Argentina.
On the Resource Website, you may download the Press Release, the
Directors’ Message, and the poster in English and in French. The poster
may be downloaded in PDF and PhotoShop formats.
Last year’s World Metrology Day was a huge success; we hope to build on
that success in 2023.
Please help us to spread the word about World Metrology Day and let the
World Metrology Day Team know by email about the events you are
organising in your country so that we can include them on the website.
For inspiration, you can see the type of events organised by other NMIs
in previous years on the website.

The ILAC MRA
As a result of the recent ILAC Arrangement Council ballot, The Southern African Development Community Cooperation in Accreditation (SADCA) has been granted recognition as a regional cooperation body of ILAC.
The scope of recognition of the recognised regions to the ILAC MRA is available from Recognised Regional Cooperation Bodies International Laboratory Accreditation Cooperation (ilac.org)
Information on the acceptance of calibration, testing and inspection results, programs provided by PTPs and reference materials produced by RMPs via the ILAC MRA is available from ILAC MRA and Signatories International Laboratory Accreditation Cooperation.
There are currently 109 signatories to the ILAC MRA representing 116 economies. The ILAC MRA covers recognition for accreditation in the areas of calibration (ISO/IEC 17025), testing (ISO/IEC 17025), medical testing (ISO 15189), inspection (ISO/IEC 17020), proficiency testing providers (ISO/IEC 17043) and reference material producers (ISO 17034). The list of signatories to the ILAC MRA is available from the ILAC MRA Signatory Search.
The ILAC MRA Annual Report 2021 and associated infographic was published in June 2022 and is available from ILAC Promotional Brochures International Laboratory Accreditation Cooperation.
The 2022 report will be published in May 2023.
Work to extend the ILAC MRA to include the accreditation of biobanks has now reached the stage where applications for recognition are being accepted. This follows the publication of the revised supporting documents as outlined in the resolution adopted during the 25th ILAC General Assembly in 2022: ILAC Resolution GA 25.09 The General Assembly endorses the recommendation of the ARC to extend the ILAC Mutual Recognition Arrangement (MRA) to include accreditation of biobanking as a Level 2 activity, to the criteria specified in the Level 3 normative document, ISO 20387. ILAC will therefore accept applications for recognition as soon as the documents within ILAC applicable to the MRA are updated to cover this new scope.
Case studies and research on the recognition of the ILAC MRA by governments and regulators are available from the Public Sector Assurance website. The website is a collaborative initiative of the INetQI members and there are over 330 case studies, 90 research papers and 60 supporting materials available to view.
The Business Benefits website a reference website designed to demonstrate the monetary value of standards, conformity assessment and accreditation for businesses. The website represents another successful collaboration of the INetQI partners with over 95 case studies categorised into 6 areas of value. All of the case studies identify a clear financial benefit. The site also includes more than 75 research papers.
ILAC Membership
The ILAC membership as of 12 April 2023 is:
The ILAC membership consists of 154 organisations from 129 different economies worldwide. Over 88,000 laboratories, 13,900 inspection bodies, 604 PTP and 260 RMP are accredited by the ILAC Full Members (signatories to the ILAC MRA).The latest statistics and graphs on the number of accreditation bodies, accredited laboratories, inspection bodies, PTPs and RMPs are available from the ILAC Facts & Figures page.
Decision to Establish a Single International Organisation for Accreditation
Work commenced on the IAF-ILAC Single Organization Project on 1 March 2021 to establish a Single International Organisation for Accreditation in accordance with the 2019 JGA Frankfurt Resolutions. The contractor managing this process is Dr.-Ing. Thomas Facklam.
The contractor is currently working closely with the Joint Steering Committee on various tasks associated with this project and in 2022 both the ILAC Extraordinary GA and the IAF/ILAC JGA focused on topics associated with this project.
ILAC Meetings
All ILAC meetings and joint ILAC-IAF meetings were again held virtually during 2022.
An extraordinary virtual meeting of the ILAC General Assembly (EGA) was held on 15 September to consider specific topics related to the establishment of the single international organisation for accreditation. The Joint IAF/ILAC General Assembly (JGA) was held virtually on 10 November 2022 and the 26th ILAC General Assembly was held virtually for the third year running on 15 November 2022. The Adopted Resolutions are available to download from the ILAC website.
The IAF/ILAC mid-term meetings are being held in Belfast, Northern Ireland, 1 – 8 May 2023. Delegates who wish to participate virtually are also able to purchase a virtual pass for these meetings. The on-site and virtual management of these meetings is being provided by Say Something Communications.
The IAF/ILAC annual meetings will be held in-person in Montréal, Canada from 6 – 15 November 2023.
In addition to the in-person meetings noted above all ILAC Committees, Working Groups and Task Forces continue to progress the items on their work plans via a combination of email and remote meetings.
Information on future meetings and events, including major regional meetings, can also be found in the ILAC Calendar.
ILAC Liaisons and other International Activities
The current list of ILAC liaison activities includes:
ILAC Liaisons with ISO and ISO/CASCO Policy Committees
ILAC Liaisons with ISO/CASCO Working Groups/Task Forces
ILAC Liaisons with Other Organisations
Information on ILAC’s partnerships, including copies of communiqués, joint procedures, press releases and MoUs, is available from the ILAC Partnerships page.
ILAC thanks all of the ILAC liaison officers, and their organisations, who volunteer their time to assist ILAC in carrying out these activities for the benefit of all ILAC members.
ILAC Secretariat
The ILAC Secretariat staff currently includes Annette Dever, Sharon Kelly, Hannah Yeoh, Stephanie Sun (0.6 FTE), Rose De Rota (0.7 FTE), and Joelle Nicolas (0.8 FTE).
ILAC Marketing and Communications Officer, Rebecca Sheehan’s last day was Friday 17 February 2023. Rebecca has taken up a permanent role that will provide greater stability and career pathways, noting that the ILAC Secretariat, in its current form will cease operation at the end of 2023. We thank Rebecca for her contribution to both ILAC and the Joint IAF/ILAC work activities while she was working with the Secretariat, and we wish Rebecca all the best for the future.

Stephanie Sun, Joelle Nicolas, Rebecca Sheehan, Hannah Yeoh, Rose De Rota, Sharon Kelly, Annette Dever
Documents and Brochures
The following publication has been finalised and released since October 2022:
ILAC-G24:2022 Guidelines for the determination of calibration intervals of measuring equipment
Follow @ILAC_Official on Twitter to receive the latest ILAC news, including information on meetings, events, liaison activities and new publications.
The April 2023 edition of ILAC News is available from News International Laboratory Accreditation Cooperation (ilac.org).
Subscribe to the latest news to receive updates from ILAC members and liaisons.
Read More
EUROLAB started 2023 with a very successful webinar on “MICROPLASTICS: regulations, standards and the role of laboratories”, on 15-16 February 2023. More than 80 participants joined us to connect, learn and share knowledge and best practices with experts in the field of microplastics, on EU legislations, standardisation activities and other key issues.
Speakers included high-level representatives from the European Chemicals Agency, European Commission DG ENV, Plastics Europe, CENELEC, ISO and the laboratory sector. Many interesting views on the definition and analysis of microplastics, standardisation initiatives and much more were presented by the speakers, with the objective to share knowledge and best practices to solve the global issue of microplastics release.
More information is available on our website, and should you be interested in the webinar contents please contact us at info@eurolab.org
 As a summary of our activities for 2022, we also recently published our Annual Report 2022, available here. The Annual Report 2022 includes an overview of EUROLAB’s most notable achievements and landmark events for last year, with details on the current and future activities, international collaborations, projects of interest to laboratories and the latest news from the Technical Committee on Quality Assurance. The document summarises EUROLAB’s involvement in the laboratory community and the cooperation with our members and stakeholders.
As a summary of our activities for 2022, we also recently published our Annual Report 2022, available here. The Annual Report 2022 includes an overview of EUROLAB’s most notable achievements and landmark events for last year, with details on the current and future activities, international collaborations, projects of interest to laboratories and the latest news from the Technical Committee on Quality Assurance. The document summarises EUROLAB’s involvement in the laboratory community and the cooperation with our members and stakeholders.
At the beginning of March, we also started our collaboration with two interesting projects: PlasticTrace and Metroracle.
PlasticTrace aims to address the urgent need for development and harmonisation of methods for the chemical identification, physical characterisation and quantification of released small micro/nanoplastics (SMPs/NPs) in drinking water, food and environmental matrices, as required by the EU’s Circular Economy Action Plan (CEAP). EUROLAB is part of the PlasticTrace Stakeholder Avisory Board.
Metroracle presents a blockchain-based concept for the digital transformation of the traceability pyramid for electrical energy measurement. It offers an innovative technical solution to requirements imposed by the digital transformation of metrology. EUROLAB and Metrolacre are collaborating on the realisation of a survey on the digitalization level and needs among laboratories.
Furthermore, our General Assembly 2023 & Accompanying Events are fast approaching. This year, the events will take place on 27-28 April 2023 in Warsaw, Poland, at the University of Warsaw Biological and Chemical Research Centre (CNBCh UW), kindly hosted by POLLAB.
It will be an important occasion not only to discuss the latest key issues for EUROLAB and the world of laboratories, but also to celebrate the 10th Anniversary of our local Member in Poland.

Finally, EUROLAB would like to invite you to the following international events that we are supporting and joining with presentations:
 |
 |
Read More

10th WORKSHOP ON PROFICIENCY TESTING IN ANALYTICAL CHEMISTRY,
MICROBIOLOGY AND LABORATORY MEDICINE
Windsor (UK), 25th – 28th September 2023
The EURACHEM Proficiency Testing Working Group (www.eurachem.org), in co-operation with CITAC (www.citac.cc) and EQALM (www.eqalm.org), is organising the 10th event of a series of Workshops addressing current practice and future directions of proficiency testing (PT) and external quality assessment (EQA) in analytical chemistry, microbiology and laboratory medicine.
Venue
The workshop will take place at the De Vere Beaumont hotel in Windsor, a town on the River Thames in southeast England, just west of London. It is home to Windsor Castle, a residence of the British Royal Family, built by William The Conqueror in the 11th century. The story of De Vere Beaumont Estate in Old Windsor is a very British one; a tale of democracy, royalty, education and religion. At its heart, sits an 18th-century mansion, a chapel, 75 event spaces and a Georgian white house in 40 acres of parkland grounds. The original house was built for Lord Weymouth but it was during its time as a public school, from 1854 – 1967, that saw most of the estate’s architectural developments.

Windsor Castle with Thames in foreground.
Technical Programme
The workshop will include training sessions, keynote lectures, short presentations, working group discussions and poster sessions, to enable interactive participation and cross-fertilisation of ideas. The official language of the workshop will be in English. Invited lectures and accepted presentations/posters will be considered, through peer-review, for publication as full papers as a topical focus in an issue of Accreditation and Quality Assurance (Springer Verlag).
Training Sessions
Four training sessions, open to workshop delegates, on the following topics:
Lectures and Working Group Topics
Who should attend?
The workshop will provide an excellent opportunity for PT/EQA scheme organisers, and end-users of PT/EQA (laboratories, accreditation bodies, regulators and the laboratories’ customers) to come together and share their views.
Registration
Visit the workshop website to register.
Workshop Secretariat
Adele Collins
Phone: +44 161 762 2555
Email: adele.collins@lgcgroup.com
Website: www.eurachem-pt2023.org
Brian Brookman
Chair Eurachem PT Working Group
LGC, Bury, UK
Read More

Growing acceptance of accreditation by regulatory bodies in UAE
United Arab Emirates (UAE) is considered one of the most progressive countries in the middle east region. UAE is leading in various fronts including scientific, educational, and economic fields in the region. The regulatory authorities and governmental conformity assessment bodies are widely adopting accreditation as a benchmark while establishing the highest levels of service standards. Various governmental conformity assessment bodies have achieved accreditation from Emirates Internal Accreditation Centre (EIAC) and other accreditation bodies to the ISO/IEC 17025, ISO/IEC 17020, ISO/IEC 17065, and ISO 15189 standards. It is worth mentioning that some of the major governmental organizations who conduct regulatory inspections have achieved ISO/IEC 17020 accreditation from EIAC such as:
EIAC accredited first Proficiency Testing (PT) provider
EIAC’s Accreditation scheme for Proficiency Testing (PT) Providers is fully functional.
The accreditation scheme was launched in 2020.
Global Proficiency Testing Company (GPTC), Dubai become the first company to receive EIAC’s accreditation according to the ISO/IEC 17043:2010 standard. GPTC’s scope of accreditation covers Water Chemistry for Sulphate, Chloride and pH Value, Water Microbiology for Legionella, TBC, Coliform, Fecal coliforms, E. Coli, Fecal Streptococci, Clostridium perfringens, Pseudomonas aeruginosa, and Construction Material (Water Proofing Membrane) for Tensile, Elongation and Thickness. The GPTC’s scope also covers Mass Calibration for Conventional Mass with Precision Weights (1 mg – 50 kg).
Ms. Amina Ahmed Mohammed EIAC CEO congratulated the management of GPTC. She said “the availability of accredited PT providers locally will enhance the prospects of conformity assessment bodies’ participation in PT programs as this would be time efficient and economical”.

EIAC CEO Ms. Amina Ahmed Mohammed is handing over accreditation certificate to Mr. Zahid Mahmood Managing Director of Global Proficiency Testing Company (GPTC), Dubai
EIAC’s accreditation scheme for medical tourism launched
The Emirates International Accreditation Centre (EIAC) formally launched an accreditation scheme for medical tourism on 9 November 2022. The scheme was launched at the DXH Partner Connect 2022 event of the Health Tourism Department of the Dubai Health Authority (DHA). Engr. Alia Ismail Al Marzouqi Director of Healthcare Sector-EIAC and Mr. Mohammad Al Muhairi Director of Health Tourism Department-DHA were present at the event. Ms. Khawla Mohamed Al Zarooni Head of Calibration Laboratories Accreditation-EIAC presented the salient features of the scheme. During Question Hour, Dr. Qasim Al Shamsi head of Healthcare Sector-EIAC explained the various aspects of the accreditation scheme.
The accreditation criteria is defined in EIAC Accreditation Standard for Healthcare Providers (EIAC-RQ-HCO-003) Annex A. The EIAC’s accreditation standard is approved by the International Society for Quality in Health Care External Evaluation Association (ISQuaEEA).

Launching ceremony of EIAC’s accreditation scheme for medical tourism
EIAC’s new accreditation scheme for certification bodies
The Emirates International Accreditation Centre (EIAC) has launched new accreditation schemes for management systems certification bodies according to ISO 17021-1 main accreditation criteria in the following areas:
The Emirates International Accreditation Centre (EIAC) has also launched a new accreditation scheme for persons certification bodies in collaboration with the Pest Control Section of Public Health Services Department of Dubai Municipality. The main accreditation criteria is ISO 17024 and the scheme is in the field of “Pest Control” for the following certifications:
EIAC attended the ARAC annual meetings & general assembly in Cairo, Egypt
The 10th annual meetings and general assembly of Arab Accreditation Cooperation (ARAC) were held from 4 – 8th December 2022 in Cairo, Egypt. A delegation from the Emirates International Accreditation Centre (EIAC) attended the annual meetings and general assembly. Ms. Amina Ahmed Mohammed CEO of EIAC who is the current chair of ARAC presided over the ARAC general assembly. Delegates from nineteen member countries and stakeholders attended the meetings. During the meetings the 10th founding anniversary of ARAC was also celebrated.

EIAC’s participation in ARAC general assembly

EIAC’s participation in ARAC general assembly
EIAC is conducting a series of trainings for the healthcare sector
The Emirates International Accreditation Centre (EIAC) is conducting a series of trainings for the healthcare sector. Four trainings are planned for assessors and laboratory staff on the ISO 15189:2022 standard and EIAC accreditation requirements.
The training facilitators are Ms. Sheila Woodcock and Mr. David Ricketts. Ms. Sheila Woodcock serves as the Convenor of ISO TC212 WG1 Quality and competence in the medical laboratory, one of the 5 Working Groups within the ISO TC212 Clinical laboratory testing and in vitro diagnostic test systems. She served as Project Leader for the revision of ISO 15189 that was published in December 2022. Mr. David Ricketts is a member of ISO TC212. He was part of the core drafting team for the revision of ISO 15189, as well as being involved in writing other laboratory standards including being the project lead for the lastest version of ISO 22870.
Two training sessions are planned for assessors for healthcare facilities including hospitals, day surgery centers, clinics, fertility centers, home health services, medical tourism and telehealth services. The training is focused on EIAC Accreditation Standard for Healthcare Providers (EIAC-RQ-HCO-003). The EIAC standard is approved by the International Society for Quality in Health Care External Evaluation Association (ISQuaEEA). Ms. Angeliki Katsapi, Dr. Alan Taylor and Dr. Bryan Woodward are the resource persons.

EIAC’s training for healthcare assessor and professionals

A fire laboratory assesses the performance of a product by testing according to a test method (notably the European test standard). Based on different measurements (e.g., temperature, radiating heat flux, etc.) but also from observations, the fire laboratory will provide the fire performance based on criteria and thresholds given in the test standard. The description of the product, the measured values, and the resulting fire performance are given by the laboratory in a fire test report.
Based on test reports, the fire laboratory issues a European classification report or national approval according to the rules specified in the classification standards.
Some National Accreditation Bodies agreed to accredit according to ExAp1 (extending the field of application) and classification standards2, however some didn’t.
ExAp application is not a test standard and is not a product standard. This was therefore discussed in relation to which is the most relevant accreditation standard covering the technical aspect of the ExAp standard. Many fire laboratories accredited according to EN ISO/IEC 17025 are also accredited as certification bodies according to EN ISO/IEC 17065. However, there are fire laboratories accredited to only EN ISO/IEC 17025 and Notified Bodies accredited only to EN ISO/IEC 17065.
EGOLF (the European Group of Organisations for Fire Testing, Inspection, and Certification) decided to support accreditation to EN ISO/IEC 17025 but asked for a common approach. As part of its harmonization mission, the EA Laboratory Committee (EA LC) established a new Task Force Group to clarify the situation.
The EA LC agreed that each laboratory can apply to be accredited by its National Accreditation Body for both Fire Classification & ExAp standards using ISO/IEC 17025 requirements on Opinions & Interpretations.
This represents a step towards harmonization (even if a NAB may decide not to develop such an activity).
EGOLF welcomed this approach as an excellent starting point since some laboratories did not previously have the possibility of accreditation for Fire classification and ExAps.
Thanks to the EA LC TFG, the way for laboratories to apply for accreditation to EN ISO/IEC 17025 and to perform assessments is now understandable and simplified.
1 In the fire testing field, you cannot test any size or characteristics of a certain product. The DiAp (Direct Field of application) given in each European test standard allows application scope directly from the fire test. The DiAp depends on the product properties and/or intended end-use applications (e.g., the size of the door leaf).
It is assumed that the same test results will be obtained if we test the item with minor differences in shape, dimension, etc.
ExAp means Extended field of Application. ExAp rules are also specified in the European standard called the ExAp standard. ExAP allows a larger application domain than DiAp.
This larger domain is accepted based on additional information. This information may come from additional tests, additional measurements during the test, and specific calculations. Those are defined in the ExAp standard (the application of these rules requires knowledge of tests standards and interrelation of the ExAp standards).
2 The classification standards define the criteria and threshold for defining the performance classification of the fire product. The input for the Fire Classification report is the test reports (including the DiAp) and ExAp reports. The existing classes are defined at the European level allowing the circulation of products. The level of performance required for a building is defined by Member states.
Read More
Forums in the form of a TV set for bodies accredited by Laboratories and Healthcare divisions
Cofrac organized two forums in the form of a TV set in less than two months. The first one, in November 2022 for the Healthcare division and the second in January 2023, for the Laboratories division.
The Healthcare division forum
After the first event organized and held face-to-face in 2018, the long awaited – since 2020 – second Healthcare forum was completely redesigned and took place in the form of a TV set, filmed and broadcast live from Paris in November 2022. The topic for this edition was “Accreditation in Healthcare: the evolutions” and was dedicated to the professionals concerned (medical biologists, ACP doctors, management, technicians, bio-medical engineers, risk managers, quality managers, …).
The programme for this half-day event included:
The event was punctuated by a live survey to collect participants’ knowledge on the upcoming version of ISO 15189 standard, a presentation of the results of a study on accreditation and medical biology laboratories led by a specialised agency and the video from the Deputy General Director of Health.
531 people were connected during the live broadcast. 78 persons answered the feedback form, with 99.9% indicating they were satisfied or very satisfied with this program and new meeting platform. Since the live broadcast, this event has been viewed more than 863 times via the replay option.
The Laboratories division forum
As with the second event for the Healthcare division, the 11th edition of “Accreditation and Laboratories” took place for the first time remotely, again filmed and broadcast live from Paris in January 2023. Some of the members of the Health division were present on the “TV set” to encourage their colleagues in this new presentation style.
This event dedicated by the Laboratories division to accredited bodies and applicants according to the ISO/IEC 17025, ISO 17034 or ISO/IEC 17043 standards gathered 530 people in the “live studio”.
This year, the program for the event included the following topics:
The event was intersperced by live surveys to collect participants’ understanding on deviation trend and seven videos. The first video was a testimony from a laboratory, and the others were Chaired by the Presidents and Vice-Presidents of the different accreditation committees who questioned the Cofrac teams in the studio. To finish there was a remote exchange with a Representative from Accredia covering EA (LC and MAC) and ILAC (AIC) international work.
119 participants answered the feedback questionnaire with 98% indicating they were satisfied or very satisfied with this program and the new meeting style. Since the live broadcast, this event has been viewed more than 998 times using the replay function.
Read More
I. ARAC MLA Signatories
Following the latest ARAC MLA Group decision ARAC has 5 MLA signatories as the following:
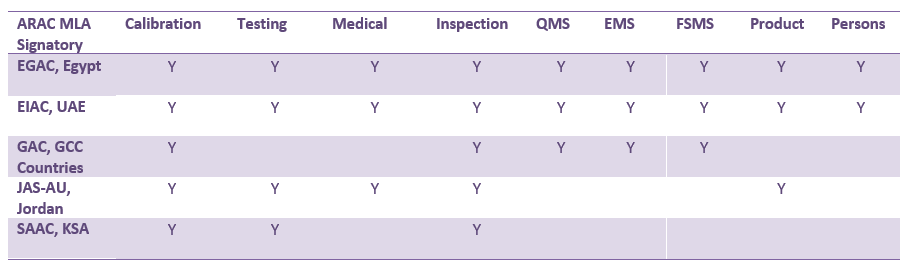
II. ARAC 10th Annual meetings:
After much planning, ARAC has held very successfully its 10th Annual Meetings in Cairo, Egypt, from 04th to 8th December 2022 hosted by the Egyptian Accreditation Council [EGAC]. The schedule for these meetings was exceptionally busy and particularly so given the significant number of Committees & Working Groups meetings and events such as the 2nd ARAC community stakeholders workshop, the celebration of ARAC 10th anniversary and the capacity building activities that included Regional training workshops on Remote Assessments/Peer Evaluations using the Augmented Reality Glasses and the key documents/records to prepare for the ARAC Peer Evaluation process.
Significant achievements were made at these meetings including the approval of the ARAC new strategy covering the period 2023-2025 and the decision to extend the ARAC MLA structure to include Proficiency Testing Providers, Reference Material Producers & Biobanking (all level 2) and Medical Devices Management System (MDMS), Information Security Management Systems (ISMS), Occupational Health & Safety Management Systems (OH&S MS) and Educational Organizations Management Systems (EOMS) (at level 4).
III. ARAC Stakeholders Community Workshop in Egypt:
The awareness stakeholder workshop was conducted on 05th December 2022 with more than 150 participants from the ARAC stakeholder community representing the conformity assessment bodies, regulatory authorities, industry & trade associations and consumer associations in Egypt.
During this workshop, the participants stressed the role that ARAC plays in the development of the accreditation and conformity assessment infrastructure in Egypt. The aim of this workshop was for stakeholders in Egypt to have a better understanding of the value of accreditation and accredited services, and in particular, for regulators to recognize and accept accredited conformity assessment results covered under the ILAC MRA, IAF MLA and ARAC MLA.
IV. ARAC New Strategy 2023-2025
ARAC members have adopted a new ARAC strategy to guide ARAC work through the period 2023-2025 with a new vision “The leading accreditation group facilitating regional and global trade and supporting sustainable development”. It is based on the 4 pillars: International recognition, Operational excellence, Cooperation & Partnership and Capabilities Development. The main objective is to strengthen all interested parties’ confidence in accreditation and enhance ARAC into a professional, efficient service provider serving the ARAC Accreditation Bodies’ Members and stakeholders.

CoverPage_ARAC Strategy2025

PATHCON & LAB EXPO 2022, an Annual Conference of the Association of Practising Pathologists, was held on the 17th and 18th of December 2022 at Hotel Taj Vivanta in New Delhi, India. Spread over two days, this unique pathology conference was organized in a hybrid mode.

This conference was attended by 623 delegates from all over the country who participated in 12 workshops and presentations by 52 faculty members. The organizing committee conducted all the sessions and workshops on the 17th. Simultaneously young and upcoming pathologists presented 120 oral papers and 132 posters that day, while on the 18th of December, senior pathologists shared their experiences in the form of exciting presentations. The discussion and deliberations covered various pathology topics, including advances in molecular diagnostics, haematology, oncology, and clinical biochemistry. They were valuable and relevant to the association members and delegates, including many postgraduate students.

One highlight of the conference was the keynote address by Dr Neeraj Jain, President of the APP, who spoke about the role of quality in pathology. He emphasized the critical importance of various quality tools for more accurate diagnoses and improving patient outcomes. Another key theme of the conference was the importance of collaboration and interdisciplinary approaches in pathology research and practice. Speakers stressed the need for pathologists to work closely with clinicians, specialists in bio-informatics and other healthcare professionals to ensure that pathology contributes to the delivery of precision medicine. The President announced a new scheme for certification of compliance with standards defined by the association. He also announced the dates of quarterly meetings and the annual Pathcon & Lab Expo 2023.

LAB EXPO 2022, the most sought-after medical laboratory exhibition in this part of India, is an integral part of PATHCON. Almost all industry partners showcase their latest laboratory equipment, devices, and technologies used in the medical field. As a result, attendees can expect to see a range of innovative laboratory tools, such as microscopes, scanners, centrifuges, auto analyzers, and other diagnostic devices. In addition, exhibitors also showcased various software solutions for data management, lab automation, and quality control. Apart from the equipment, the Lab Expo provided an opportunity to attend seminars and workshops presented by industry experts, offering attendees a chance to update their knowledge on recent developments and techniques in the field. Lab Expo also provided an opportunity to exchange knowledge and ideas and create an interface between laboratory medicine professionals and the industry.

Overall, this Pathcon and Lab Expo 2022 was a grand success and provided an excellent opportunity for members of the association and delegates to be updated on the latest trends and advancements in medical laboratory technology.
Read More