
Accredited testing laboratories for the construction and civil works sector in Colombia.
By: Paola Aguirre V., Diana Jácome M., and Mauricio Rodríguez R. International Technical Direction. National Accreditation Body of Colombia – ONAC
Let’s start with a little history about accredited laboratories in Colombia.
When we talk about accredited laboratories, it is important to highlight the fact that the first accreditation granted by ONAC in laboratories scheme, was to a construction and civil works one. It was in 2009 (14 years ago), the same year ONAC started with the accreditation activity in the country.
Today, this is the sector with the highest number of accredited testing laboratories (52) that represents 19% of the total number of accredited testing laboratories. Thus, since 2019 a growth trend has been evidenced in this sector of laboratories, as identified in Graph 1, the highest growth rate was achieved in 2021, reaching 10 new accredited laboratories. Moreover, the first semester of 2023, accreditation has been granted to 3 new laboratories and there are another 5 in the process of accreditation, maintaining the growth trend that started since 2019.
In addition, it is worth mentioning that since 2014, the testing laboratories scheme has the international recognition signed with the InterAmerican Accreditation Cooperation (IAAC) and the International Laboratory Accreditation Cooperation (ILAC), which are multilateral agreements that allows around 100 economies worldwide to accept and recognize the results issued by testing laboratories accredited by ONAC.
Graph 1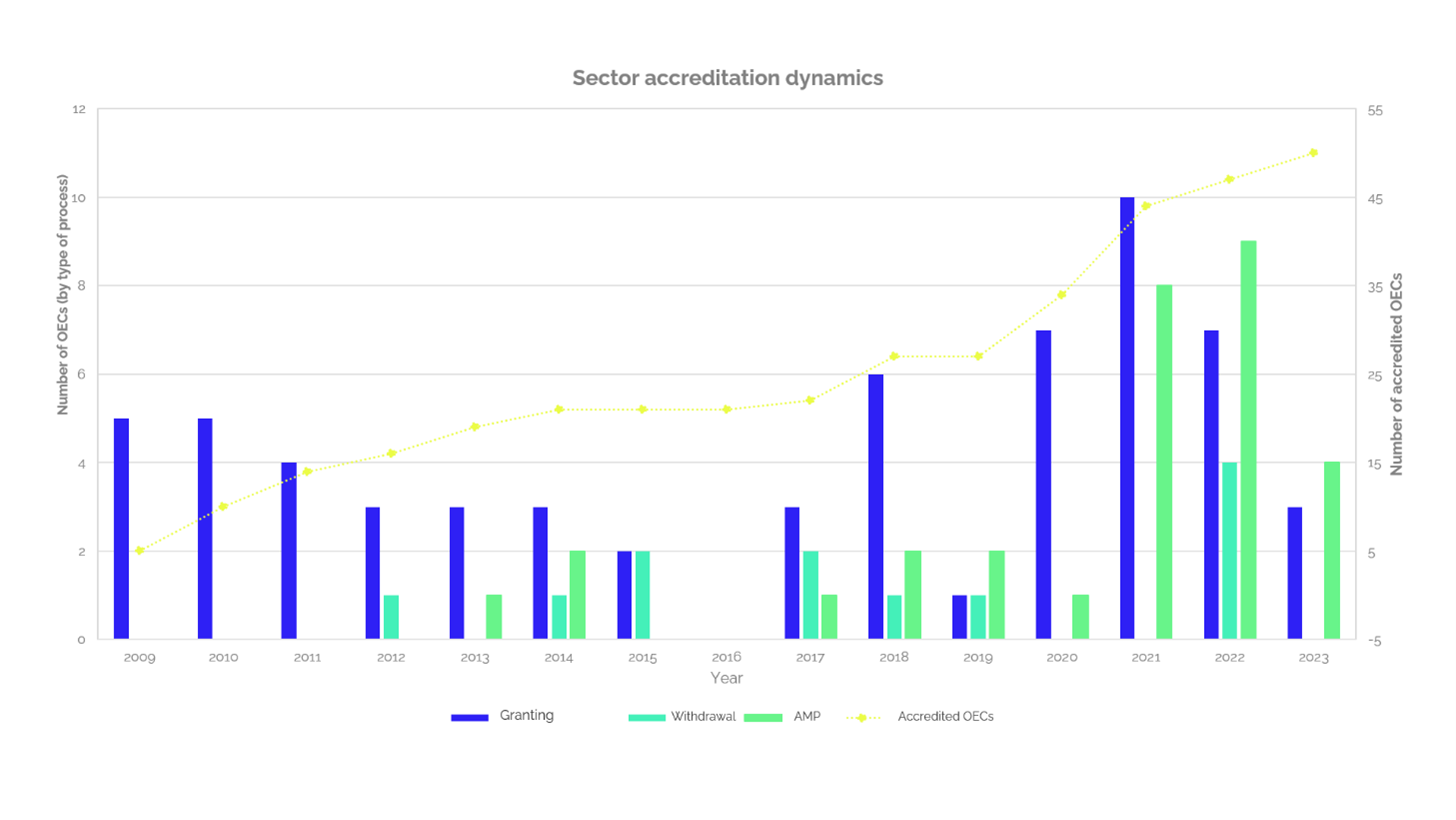
Additionally, Graph 1 also shows the number of new laboratories accredited pr year (grants), the increase in the number of testing or the number of sites (expansion), laboratories that have had their accreditation withdrawn (withdrawals), and the net number of accredited laboratories in the sector. Thus, since the first accredited laboratory, the sector achieves an average growth rate of 3.3 new laboratories per year.
Regarding the withdrawals of accreditation, no clear trend is observed, these are considered isolated cases, with a maximum of 4 for 2022, 2 in the years 2015 and 2017, and values of 1 or 0, for the other years. On the other hand, in the last years there is also an increasing trend regarding the expansion of accreditation, taking into account that only until 2013 the first expansion of the scope was presented by one laboratory accredited in the construction sector, with values between 1 or 2 laboratories that expanded their accredited scope between 2013 and 2020.
In the last two years there was a substantial increase in applications, 8 laboratories in 2021, 9 laboratories in 2022 and 4 so far in 2023 have expanded their accredited scope, showing that not only in the last two years the number of accredited laboratories in the sector has increased, but that laboratories are seeking to expand the portfolio or capacity of their accredited services to meet market needs.
The fact that the number of accreditations for testing laboratories in this sector is predominant with respect to the other sectors of laboratories accredited by ONAC, demonstrates the importance and confidence that the user of these services, recognize in accreditation, which is a fundamental element to confirm the competence to guarantee the validity of the results that are issued, from which relevant decisions will be taken within the processes of planning and execution of the constructions and civil works.
Overview of accredited laboratories
When we talk about accredited laboratories, it is important to keep in mind which is the scope of each accreditation, i.e., which are the specific activities that each laboratory performs competently. These scopes are dynamic and depend on the services that each laboratory has demonstrated that can perform competently, including, in effect, the traceability of its measurements to the International System of Measurements (SI).
One of the aspects that are part of the accredited scope are the laboratory locations, from which three types of laboratories can be differentiated: permanent, on-site and mobile laboratories. The first ones, are those that perform the tests in the permanent location of the laboratory, which is clearly registered in the accredited scope and using equipment that cannot be moved from one location to another.
The second category is characterized because the tests are performed with portable equipment, directly at the sampling site, which may be at the front of the construction or civil works, delivering results immediately in most cases. And the last one, it is the complete laboratory that moves to the sampling site, regardless of the nature of the equipment used in the tests. Each type of laboratory presents a series of conditions, restrictions and benefits when providing services, but, as long as they are covered by the accreditation granted by ONAC, they comply with the elements of competence established in the international standard ISO/IEC 17025 and the requirements of the International Laboratory Accreditation Cooperation (ILAC).
Currently, ONAC has 52 accredited Conformity Assessment Bodies (CABs) for this sector, legal entities that subscribe the accreditation with ONAC. The operational headquarters or Laboratories reach a total of 67 (permanent site) covering 11 departments from 32 in whole country, and 20 cities and/or municipalities, information that can be identified in Figure 1. Additionally, fourteen (14) CABs have laboratories that are accredited to perform on-site testing and one (1) corresponds to a mobile laboratory, being this the only sector that has this type of laboratory.
Graph 2
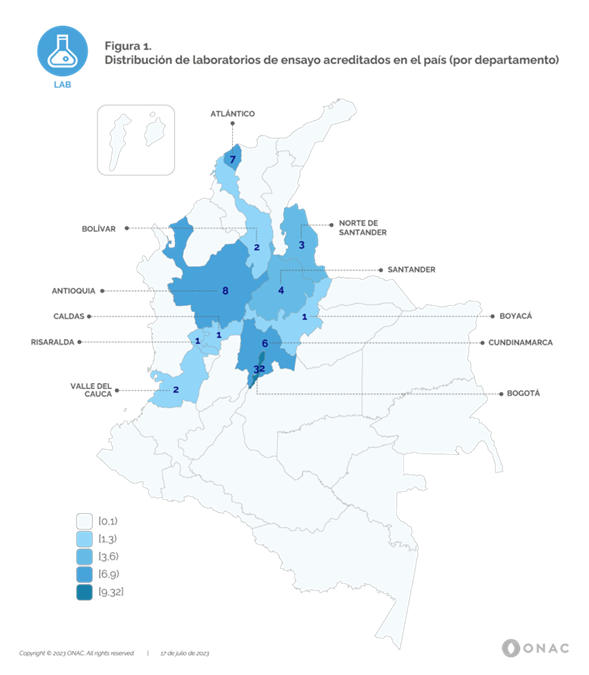
As can be seen in Figure 1, the largest number of accredited laboratories are located in the city of Bogotá, D.C., with 32 laboratories, equivalent to 48% of the total, Antioquia has 8 laboratories, representing 12%, Atlántico with 7 laboratories, reaches 10%, Cundinamarca with 6 laboratories equals 9%, Santander with 4 laboratories accredited laboratories reaches 6%, Norte de Santander with 3 laboratories reaches 5%, Valle del Cauca and Bolívar with 2 laboratories, 3% each one and Risaralda, Caldas and Boyacá with 1 laboratory, correspond to 1.5% each one. In general terms, testing activities for the sector are centralized in the departments of the Andean, Caribbean and Pacific regions, covering the main capital cities. It is important to clarify that the 14 on-site laboratories and the mobile laboratory cover the entire national territory.
When we refer to the scopes accredited in Construction laboratories, it is possible to analyze them from several aspects, starting with the type of testing provided, as shown in Graph 2, in which tests under mechanical techniques predominate, reaching 40% of the total accredited tests offered by the accredited laboratories, tests that include: tensile, tension, breakage, compression, among others, to determine material resistance.
Graph 3
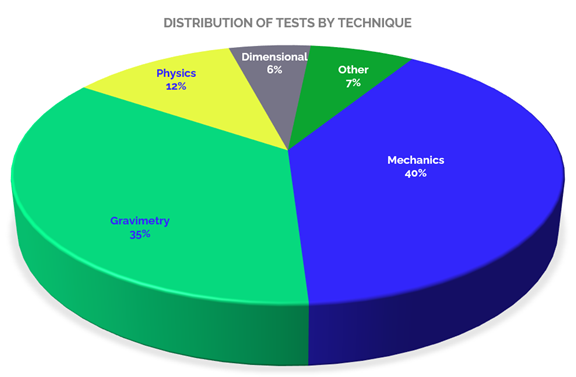
Secondly, there are gravimetric techniques, which constitute 35 % of the total number of accredited tests, including: moisture determination and ratios, particle size distribution, aggregate index, density, among others. 12% corresponds to physical tests, which include the determination of surface quality, linear mass, moisture content by distillation, among others. 6 % of the accredited tests are dimensional, with tests such as: measurement of projections in bars, thickness of compacted specimens of asphalt mixtures, measurement of graphite and wires, among others.
And finally, the remaining 7% (Others) corresponds to tests performed under various techniques such as: physicochemical, colorimetry, optical emission spectrophotometry, rheology, among others. All these tests are of great relevance for the sector, as they allow to determine the quality of materials and items, and thus guarantee their reliability and adequacy in the construction processes.
Another way to classify the accredited scopes of the sector’s laboratories corresponds to the test item, although a laboratory may have within its portfolio, for example, tests in the mechanical field, this is limited to a detailed group of items, for which it has demonstrated that it can provide services competently. Graph 3 shows the distribution of accredited tests by type of item.
Graph 4
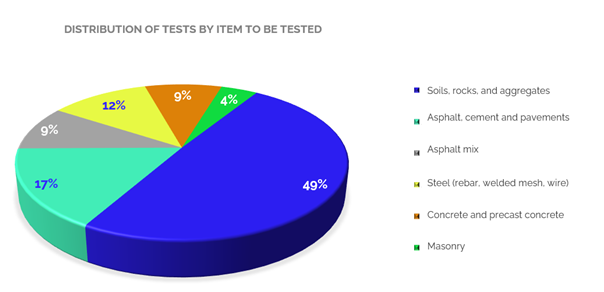
The accredited tests cover several types of items and materials. Among these, we find tests for soils, rocks and aggregates (fine and coarse aggregates), which are mostly gravimetric and the most offered in the sector, reaching 49 % of the total accredited scopes. Then, with 17 % are the tests performed on asphalt, cement and pavements, including hydraulic cement, and with 9 % the tests on asphalt mixtures, in which for these two types of materials the tests are predominantly of mechanical and gravimetric type.
Accredited tests on steel materials (rebar, smooth bars, electro welded mesh, smooth and drawn steel wire, among others) represent 12 % of the total number of tests, and for this type of materials, most of the tests are mechanical. Tests on concrete (including hydraulic concrete) and prefabricated concrete, in which mechanical compression and flexural tests predominate, represent 9 %, and tests on masonry materials (paving blocks, slabs, bricks, blocks, etc.) of mechanical type represent 4 %.
Although the materials and items mentioned above are those that predominate in the construction and civil works sector, there are also accredited testing laboratories that offer services for complementary materials and items or items of indirect use for the sector, such as electrical elements, plastics, pipes, among others. All these scopes and the accredited laboratories that provide these services can be consulted in the Official Directory of Accredited Laboratories – DOA.
If we analyze the accredited scopes by type of standard or testing standards used for the provision of services, we can identify a distribution as shown in Graph 4. In which we can see that 47% of the tests offered by accredited laboratories in this sector are performed according to the guidelines of the standards of the National Roads Institute (INVIAS), which is probably related to the needs generated by the institutions that outline the requirements in the contracting of public civil works.
Graph 5
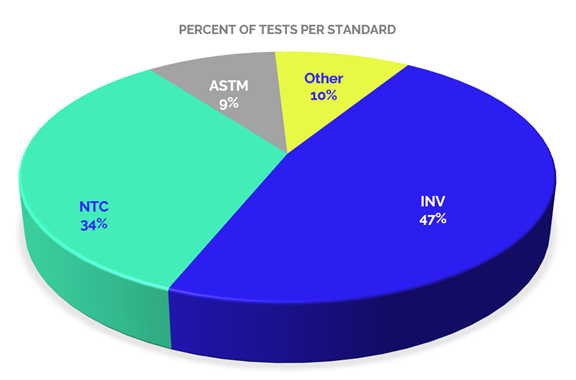
While 34 % of the accredited tests are performed under National Technic Colombian standard – NTC standards, issued by ICONTEC, recognized by the Colombian Government as the National Standardization Organization, these standards are usually adoptions of international standards or standards developed by national standardization committees. In a low percentage, 9%, there are tests with ASTM international standards, and 10% with other standards such as AASHTO (American Association of State Highway and Transportation Officials), API (American Petroleum Institute), among others.
Considering that almost half of the tests are performed with INVIAS standards, documents based on NTC standards, which are derived from international standards such as ASTM, it is necessary to call attention to the fact that these standards are outdated in relation to the international documents on which they are based (mainly 2013).
This is due to the fact that the speed with which international standards are produced and updated is greater than the speed with which these documents are adopted in Colombia; even more so, when they are included in National Technical Regulations or in the terms for contracting of these services by the State. This lack of updating represents a competitive disadvantage for national laboratories in relation of the mutual recognition agreements that ONAC has signed with ILAC.
Finally, from the information available in ONAC, it is possible to identify the type of organization that owns the accredited laboratories. Most of the accredited laboratories in the sector correspond to private entities, reaching 85%, being clear that it is this type of entities that can adjust their strategies, budgets and operations in a more dynamic way to the market demand in this economic sector. The remaining 15% of the laboratories correspond to public entities. Now, considering the importance of this sector in a developing country like ours, it is highlighted that the academy has taken part in this sector, 9 laboratories are identified ascribed to educational institutions, reaching a participation of 17 %, three laboratories of SENA (National Learning Service) and 6 laboratories of universities, of which two correspond to public universities.
Some reflections
The analysis carried out allows identifying the growth trend of accredited testing laboratories for the construction and civil works sector, as well as some classifications that can be made based on the accredited scopes and the legal nature of the accredited laboratories, but it also allows showing the areas, specific sectors, techniques, items or materials, where there is no supply or the existing supply is insufficient.
An economic and growth sector, such as construction and civil works, in terms of infrastructure for a country owes its success, growth and contribution to society, in part to the quality assurance of its processes, as well as the search for efficiency in its construction methods, for which accredited conformity assessment services, such as the activities performed by testing laboratories, are fundamental and indispensable, in the different stages, from planning, to the confirmation of designs and compliance with specifications once the works or constructions are completed.
For this reason, having a greater capacity of accredited testing, with a greater offer and with the globally accepted level of competence, is a strategic aspect in the competitiveness of the sector.

2. Conferences

The inspection division of Cofrac in now offering a new accreditation scheme for the control and verification of removable structures to ensure the safety of people.
What is a removable structure?
A removable structure is a temporary and dismountable structure for sporting, cultural, commercial, or tourist events and consists of a framework that can be repeatedly assembled and dismantled for temporary use.
Removable structures are divided in two groups:
Context and challenges of this accreditation scheme
In France, until 2022, there were no rules for verifying the mechanical strength of demountable structures, or for assessing an organization’s ability to control and verify them. However, the industry had created a practical guide that the safety commissions also relied on.
With the Paris 2024 Olympics Games approaching and safety issues at meetings associated with major gatherings, it is necessary to fill the regulatory gap.
A working group has been set up by the French Ministry of the Interior and Overseas Territories to draw up a technical reference system setting out the design, installation and operating rules, as well as the inspection and verification methods for these structures. This work was based on the “Practical Guide
– Dismantable Equipment and Assemblies” drawn up in 2017 by SYNAPSE and the “Good Practice Guide
– Dismantable Equipment and Assemblies” of the Paris Police Prefecture.
Cofrac was also involved in this work due to the Ministry’s decision to rely on accredited organizations to carry out these inspections.
What are these checks and inspections?
Controls and checks are divided into two phases:
On October 1, 2022, Cofrac launched the accreditation scheme according to the NF EN ISO/IEC 17020 standard for two areas of inspection: the design control and the verification of assembly and operational inspection. The assessment plan relies on the decree published on August 5, 2022, setting out the safety rules, technical provisions, inspection control obligations and conditions applying to these temporary and dismountable structures.
This scheme has two specificities:
Accreditation is mandatory for organizations that are not approved technical inspectors. It therefore constitutes a real opportunity for these organizations to be able to provide this type of service, in view of their broader markets and skills.
Accreditation already existed for similar activities such as scaffolding, mechanical shelving and structural strength testing. To meet the demand for accreditation, Cofrac extended the qualifications of some of these technical assessors to these new schemes and has also qualified new assessors.
To date, 4 organizations are accredited for these new schemes, and others are in the process of becoming accredited.
Accreditation will be mandatory from 1 January 2024 for all inspection bodies wishing to operate in this technical field.

EIAC celebrated World Accreditation Day
The Emirates International Accreditation Centre (EIAC) celebrated world accreditation day. In line with this year’s theme “Supporting the Future of Global Trade” EIAC arranged various activities with the collaboration of stakeholders and regulatory authorities. The main event was held in the JW Marriot Marquis hotel in Dubai. The event was attended by representatives from accredited conformity assessment bodies, Ministry of Industry and Advanced Technology, UAE, Dubai Health Authority, Food Safety Department and industry. Addressing the audience, Ms. Amina Ahmed Mohammed, the Chief Executive Officer of the Emirates International Accreditation Centre (EIAC), highlighted the importance of accreditation and the role the International Laboratory Accreditation Cooperation (ILAC) and International Accreditation Forum (IAF) play in supporting global trade. She said the future of global trade is expected to be technology driven where globally recognized standards will have utmost importance. Dr. Farah Al Zarooni, Assistant Undersecretary of UAE Ministry of Industry and Advanced Technology, addressed the gathering saying UAE in general and Dubai specifically, have become a global hub for trading. She highlighted the various government initiatives in supporting global trade through developing standardized and efficient inspection methods and adopting technological advancement in ports and shipping operations. A panel discussion titled “from local to global” was also part of the event.

EIAC CEO Ms. Amina Ahmed Mohammed addressing the World Accreditation Day celebrations
Safety of Fairground & Amusement Equipment is paramount
In 2022, the UAE has seen significant growth in the travel and tourism sector that has contributed to 9% of the total GDP, while Dubai alone has seen a growth of 53.8% compared to 2021. According to “Euromonitor’s Top 10 City Destinations Index 2022”, Dubai was ranked the second most visited city in the world.
Addressing a tourism seminar in Dubai, Ms. Amina Ahmed Mohammed, the Chief Executive Officer of the Emirates International Accreditation Centre (EIAC), said Dubai is a popular destination for amusement parks equipped with advanced indoor and outdoor amusement equipment, serving millions of visitors yearly. Other Emirates of the United Arab Emirates (UAE) including Abu Dhabi are also home of various branded theme parks. Thus, safety of fairground and amusement equipment used in these parks is paramount. She said the EIAC’s accreditation schemes for inspection bodies in Fairground and Amusement Equipment provides assurances to the public about the safety of amusement rides and play areas. Ms. Amina Ahmed Mohammed said EIAC also has an accreditation scheme for the certification of persons who are working in the amusement parks and fairgrounds, that further enhances the prospects of safe operations in the sector. It was further noted that the EIAC’s accreditations are globally recognized.
The leisure and entertainment sector plays a vital role in achieving the ‘UAE Tourism Strategy 2031’ that aims to significantly increase the tourism sector’s contribution to the nation’s economy.
Addressing the seminar, Engr. Yousef Ahmed Aljasmi, the Director of Inspection Bodies Accreditation Department of EIAC, provided input on EIAC’s plans in reinforcing the quality infrastructure of the Leisure and Entertainment sector by continually supporting the development of the local Inspection and testing bodies in collaboration with regulators and stakeholders. Engr. Yousef said the EIAC’s accreditation scheme covers the design review, manufacturing process, initial and in-service examinations and inspections.

Engineer Yousef Ahmed Al Jasmi addressing the seminar
EIAC’s unique and new accreditation scheme for certification bodies
The United Arab Emirates (UAE) is the home of people from more than 200 nationalities. The concepts of tolerance and coexistence in the UAE are considered fundamental in maintaining social harmony and peace based on cultural pluralism, accepting others, and rejecting discrimination, hatred, and intolerance in society. The UAE has issued UAE Standard UAE.S 5037: 2021 “Tolerance and Coexistence” for promotion of tolerance and coexistence.
The Emirates International Accreditation Centre (EIAC) has launched a new accreditation scheme for management systems certification bodies according to ISO 17021-1 main accreditation criteria for UAE S. 5037: 2021 “Tolerance and Coexistence management systems.
The Emirates International Accreditation Centre (EIAC) is also working to launch a new accreditation scheme for persons certification bodies in collaboration with Health and Safety Department of Dubai Municipality. The main accreditation criteria is ISO 17024 and the scheme is for the certification of “Health and Safety officers for labor accommodations”.
EIAC attended IAF-ILAC and ARAC mid-term meetings
Delegations of Emirates International Accreditation Centre (EIAC) attended the mid-term meetings of IAF-ILAC and ARAC. THE IAF-ILAC mid-term meetings were held in Belfast, Northern Ireland and the ARAC mid-term meetings were held in Manama, Bahrain. Ms. Amina Ahmed Mohammed, CEO of EIAC, and the current chair of ARAC lead the EIAC delegations. Delegates from member bodies attended the meetings.

EIAC’s delegation attended IAF-ILAC and ARAC mid-term meetings
EIAC conducted various trainings for laboratories and inspection bodies
The Emirates International Accreditation Centre (EIAC) has conducted various trainings for laboratories and inspection bodies in specialized sectors.
A special workshop for the accredited Inspection Bodies in accordance with ISO/IEC 17020 & ILAC P15 was conducted. The target audience of the workshop was the inspection bodies that are working in the field of inspections of Fairground and Amusement Equipment for the scope of independent (in-service) examination. The aim of this workshop was to enrich the market with locally based Inspection Bodies to sustain the continuity of businesses managing a variety of complex attractions in Dubai, Abu Dhabi and other emirates.
ISO/IEC 17025 training for experts of the Federal Authority for Nuclear Regulation (FANR) was conducted in Abu Dhabi in June. Mr. Ahmed Saad Was the resource person.

EIAC’s training for inspection bodies for amusement parks and fairgrounds inspection

EIAC’s training on ISO/IEC 17025

EIAC’s training on ISO/IEC 17020
Training on proficiency testing was conducted in July in Dubai with Mr. Mohammad Saaed as the resource person.
Training for medical laboratories was conducted in September and Dr. Venkatesh Thuppil was the main resource person.

Municipal pipelines such as water supply and drainage, electricity, gas and communication are buried under urban roads which are affected by vehicle load, vibration, groundwater level and other factors. Disasters such as pavement collapse and cracking, are easily caused which result in inconvenience to urban production and life and may even cause personal injury. This is particularly the case in recent years, with the further expansion of the urban scale and the increasing density of underground pipe network in some cities. Geological disasters of urban roads are occurring frequently, which has attracted the increased attention of the municipal departments and the general public.
Similarly, the application of robot technology in the inspection of internal defects of urban sewer and non-excavation pipeline repair technology has attracted wide attention because of the advantages of high repair efficiency and low repair cost.
After a period of service, the materials used in urban roads and urban sewers gradually age and their functions decline potentially resulting in geological disasters and potential safety hazards. In order to find the potential safety hazards and take measures to eliminate them, it is necessary to regularly evaluate the risk of urban road underground failures and the internal defects of urban sewer. Since accreditation is an affirmation of the management and technical competence of the inspection body, there is an increasing demand for accreditation in the competence of risk evaluation in underground failures related to urban roads and the competence in internal defect evaluation of urban sewers.
CNAS has actively responded to the accreditation needs in the above fields organizing experts to study and discuss the characteristics and technical requirements of inspection and testing activities in these two fields, and promote accreditation of the inspection bodies. Following analysis, the inspection items for urban roads is the detection and evaluation of attributable characteristics of underground failures such as cavities underneath the pavement, voids, loosely infilled voids, water-rich voids in the area using detection methods such as ground penetrating radar method for urban roads according to relevant technical standards, and to carry out a risk evaluation. The inspection items for urban sewers are sewer defects and condition evaluation, including the detection of structural and functional defects of urban sewers using closed circuit television inspection and quick view pipe inspection methods using the relevant technical standards Then to evaluate and judge the structural and functional conditions of the sewer and calculate the rehabilitation index and maintenance index. These two types of activities include the typical characteristics of inspection activities. Therefore, inspection accreditation in accordance with ISO/IEC 17020 is applicable.
CNAS is accrediting inspection bodies in these two technical fields. Competence in the detection and evaluation of underground failures in urban roads and sewer defect and condition evaluation of urban sewers is accredited according to the inspection body accreditation standard ISO/IEC 17020. As of August 2023, in the above fields, a total of 31 inspection bodies have been accredited by CNAS, with more inspection bodies currently in the process of seeking accreditation for these activities.

Reference laboratories are not referred to in the context of accreditation as often as calibration or testing laboratories. However, during COVID-19 epidemic, it was reference laboratories that came into the focus of increased public attention due to stories about the possibility of their production of dangerous bacteria and viruses, which at some point, intentionally or accidentally, can break out of the laboratory walls. This reputational risk was unexpectedly raised in relation to the reference laboratories of Kazakhstan potential involvement in the development of biological weapons. As a full member of ILAC, the National Center of Accreditation (NCA) of the Republic of Kazakhstan would like to tell you about how the activities of reference laboratories are regulated in our country.
Kazakhstan is an exceptionally peaceful country, where more than 100 nationalities live in harmony on a large territory. Commitment to everything that contributes to the continuation of this peaceful life is not just a national ideology – it is woven into the consciousness and subconsciousness of Kazakhstanis. Therefore, any accusations of unseemly actions against human civilization are perceived very painfully in our country.
The history of 2020 ended well when diplomats and the scientific community of our country explained to the public how and what reference laboratories actually work on, and that their activities are kept in strict accordance with the scope of accreditation, that is, exclusively for medical and civil purposes.
There are seven reference laboratories in Kazakhstan. Their listing is approved by the order of the Minister of Health of Kazakhstan, and it is impossible to get into this list without permission and control.
In the Council of Europe, reference laboratories are structural divisions of the Ministry of Health of Kazakhstan, and the tasks that they solve are carried out under the control of the Ministry.
In the Council of Europe, reference laboratories of Kazakhstan are accredited entities under the national standard of ST RK ISO 15195-2018 “Laboratory medicine. Requirements for reference measurement laboratories”, identical to the international standard ISO 15195:2018 Laboratory medicine – Requirements for reference measurement laboratories. Of course, this is not a new standard for the world of standardization, but given 30-years of experience as an independent state, Kazakhstan sees it as a good tool for improving the country’s healthcare system.
Thus, as an accreditation center, we would like to once again remind you of the importance of reference laboratories that focus on studying microorganisms to protect humanity from possible harm.

The Saudi Accreditation Center (SAAC) held the World Accreditation Day Forum 2023 on June 8, 2023, in Riyadh with its third edition, under the theme “Accreditation and Promoting the Future of International Trade”. The event witnessed the attendance of several distinguished guests, officials, representatives from both the government and private sectors, in addition to numerous experts in the accreditation field.
Dr. Adel bin Abdul Rahman Al-Qaid, the Executive Director of the Saudi Accreditation Center, opened the ceremonies with a welcoming speech, in which he emphasized the importance of accreditation in promoting international trade, and the role of quality infrastructure in establishing reliable local and global supply chains.
The forum held two discussion sessions: starting with “the Quality of Saudi Industry as Insurance to Accessing Global Markets”, which addressed the active role of accreditation bodies in increasing the appeal of local products and promoting them in different markets, while the second session entitled “the Role of Quality in Supporting Global Supply Chains” discussed the efficiency of establishing a local infrastructure that’s capable of supporting local supply chains to become an integral part of the global supply network.
The Saudi Accreditation Center always ensures that World Accreditation Day is commemorated, in conjunction with the International Accreditation Forum “IAF”, the International Laboratory Accreditation Organization “ILAC”, as well as international accreditation bodies around the world. This is based on the strategic objectives of SAAC to enhance the quality infrastructure in the Kingdom of Saudi Arabia to reach the top 10 globally by 2030.
It’s worth mentioning that the forum witnessed a significant attendance of 400 registered participants, and the sessions hosted 8 speakers, including 5 government representatives, and 3 from private companies.




The Czech Accreditation Institute (CAI) was established as a national accreditation body by the Czech Republic on 1st January 1993. In January 2023, a festive meeting of CAI staff was held to celebrate the 30th anniversary. The meeting took place in the splendid surroundings of the historic town of Jindřichův Hradec, founded by the royal family of the Přemyslid in the 10th century.

Participants recalled the history of the establishment and operation of the CAI from its earliest beginnings to the present day.
In 1996 the initial peer-evaluation of CAI was undertaken and in 1998 the CAI signed the EA Multilateral Agreement (MLA) to become the first EA MLA signatory in Central and Eastern Europe and in doing so gained international recognition.

Growing acceptance of accreditation by regulatory bodies in UAE
United Arab Emirates (UAE) is considered one of the most progressive countries in the middle east region. UAE is leading in various fronts including scientific, educational, and economic fields in the region. The regulatory authorities and governmental conformity assessment bodies are widely adopting accreditation as a benchmark while establishing the highest levels of service standards. Various governmental conformity assessment bodies have achieved accreditation from Emirates Internal Accreditation Centre (EIAC) and other accreditation bodies to the ISO/IEC 17025, ISO/IEC 17020, ISO/IEC 17065, and ISO 15189 standards. It is worth mentioning that some of the major governmental organizations who conduct regulatory inspections have achieved ISO/IEC 17020 accreditation from EIAC such as:
EIAC accredited first Proficiency Testing (PT) provider
EIAC’s Accreditation scheme for Proficiency Testing (PT) Providers is fully functional.
The accreditation scheme was launched in 2020.
Global Proficiency Testing Company (GPTC), Dubai become the first company to receive EIAC’s accreditation according to the ISO/IEC 17043:2010 standard. GPTC’s scope of accreditation covers Water Chemistry for Sulphate, Chloride and pH Value, Water Microbiology for Legionella, TBC, Coliform, Fecal coliforms, E. Coli, Fecal Streptococci, Clostridium perfringens, Pseudomonas aeruginosa, and Construction Material (Water Proofing Membrane) for Tensile, Elongation and Thickness. The GPTC’s scope also covers Mass Calibration for Conventional Mass with Precision Weights (1 mg – 50 kg).
Ms. Amina Ahmed Mohammed EIAC CEO congratulated the management of GPTC. She said “the availability of accredited PT providers locally will enhance the prospects of conformity assessment bodies’ participation in PT programs as this would be time efficient and economical”.

EIAC CEO Ms. Amina Ahmed Mohammed is handing over accreditation certificate to Mr. Zahid Mahmood Managing Director of Global Proficiency Testing Company (GPTC), Dubai
EIAC’s accreditation scheme for medical tourism launched
The Emirates International Accreditation Centre (EIAC) formally launched an accreditation scheme for medical tourism on 9 November 2022. The scheme was launched at the DXH Partner Connect 2022 event of the Health Tourism Department of the Dubai Health Authority (DHA). Engr. Alia Ismail Al Marzouqi Director of Healthcare Sector-EIAC and Mr. Mohammad Al Muhairi Director of Health Tourism Department-DHA were present at the event. Ms. Khawla Mohamed Al Zarooni Head of Calibration Laboratories Accreditation-EIAC presented the salient features of the scheme. During Question Hour, Dr. Qasim Al Shamsi head of Healthcare Sector-EIAC explained the various aspects of the accreditation scheme.
The accreditation criteria is defined in EIAC Accreditation Standard for Healthcare Providers (EIAC-RQ-HCO-003) Annex A. The EIAC’s accreditation standard is approved by the International Society for Quality in Health Care External Evaluation Association (ISQuaEEA).

Launching ceremony of EIAC’s accreditation scheme for medical tourism
EIAC’s new accreditation scheme for certification bodies
The Emirates International Accreditation Centre (EIAC) has launched new accreditation schemes for management systems certification bodies according to ISO 17021-1 main accreditation criteria in the following areas:
The Emirates International Accreditation Centre (EIAC) has also launched a new accreditation scheme for persons certification bodies in collaboration with the Pest Control Section of Public Health Services Department of Dubai Municipality. The main accreditation criteria is ISO 17024 and the scheme is in the field of “Pest Control” for the following certifications:
EIAC attended the ARAC annual meetings & general assembly in Cairo, Egypt
The 10th annual meetings and general assembly of Arab Accreditation Cooperation (ARAC) were held from 4 – 8th December 2022 in Cairo, Egypt. A delegation from the Emirates International Accreditation Centre (EIAC) attended the annual meetings and general assembly. Ms. Amina Ahmed Mohammed CEO of EIAC who is the current chair of ARAC presided over the ARAC general assembly. Delegates from nineteen member countries and stakeholders attended the meetings. During the meetings the 10th founding anniversary of ARAC was also celebrated.

EIAC’s participation in ARAC general assembly

EIAC’s participation in ARAC general assembly
EIAC is conducting a series of trainings for the healthcare sector
The Emirates International Accreditation Centre (EIAC) is conducting a series of trainings for the healthcare sector. Four trainings are planned for assessors and laboratory staff on the ISO 15189:2022 standard and EIAC accreditation requirements.
The training facilitators are Ms. Sheila Woodcock and Mr. David Ricketts. Ms. Sheila Woodcock serves as the Convenor of ISO TC212 WG1 Quality and competence in the medical laboratory, one of the 5 Working Groups within the ISO TC212 Clinical laboratory testing and in vitro diagnostic test systems. She served as Project Leader for the revision of ISO 15189 that was published in December 2022. Mr. David Ricketts is a member of ISO TC212. He was part of the core drafting team for the revision of ISO 15189, as well as being involved in writing other laboratory standards including being the project lead for the lastest version of ISO 22870.
Two training sessions are planned for assessors for healthcare facilities including hospitals, day surgery centers, clinics, fertility centers, home health services, medical tourism and telehealth services. The training is focused on EIAC Accreditation Standard for Healthcare Providers (EIAC-RQ-HCO-003). The EIAC standard is approved by the International Society for Quality in Health Care External Evaluation Association (ISQuaEEA). Ms. Angeliki Katsapi, Dr. Alan Taylor and Dr. Bryan Woodward are the resource persons.

EIAC’s training for healthcare assessor and professionals

Forums in the form of a TV set for bodies accredited by Laboratories and Healthcare divisions
Cofrac organized two forums in the form of a TV set in less than two months. The first one, in November 2022 for the Healthcare division and the second in January 2023, for the Laboratories division.
The Healthcare division forum
After the first event organized and held face-to-face in 2018, the long awaited – since 2020 – second Healthcare forum was completely redesigned and took place in the form of a TV set, filmed and broadcast live from Paris in November 2022. The topic for this edition was “Accreditation in Healthcare: the evolutions” and was dedicated to the professionals concerned (medical biologists, ACP doctors, management, technicians, bio-medical engineers, risk managers, quality managers, …).
The programme for this half-day event included:
The event was punctuated by a live survey to collect participants’ knowledge on the upcoming version of ISO 15189 standard, a presentation of the results of a study on accreditation and medical biology laboratories led by a specialised agency and the video from the Deputy General Director of Health.
531 people were connected during the live broadcast. 78 persons answered the feedback form, with 99.9% indicating they were satisfied or very satisfied with this program and new meeting platform. Since the live broadcast, this event has been viewed more than 863 times via the replay option.
The Laboratories division forum
As with the second event for the Healthcare division, the 11th edition of “Accreditation and Laboratories” took place for the first time remotely, again filmed and broadcast live from Paris in January 2023. Some of the members of the Health division were present on the “TV set” to encourage their colleagues in this new presentation style.
This event dedicated by the Laboratories division to accredited bodies and applicants according to the ISO/IEC 17025, ISO 17034 or ISO/IEC 17043 standards gathered 530 people in the “live studio”.
This year, the program for the event included the following topics:
The event was intersperced by live surveys to collect participants’ understanding on deviation trend and seven videos. The first video was a testimony from a laboratory, and the others were Chaired by the Presidents and Vice-Presidents of the different accreditation committees who questioned the Cofrac teams in the studio. To finish there was a remote exchange with a Representative from Accredia covering EA (LC and MAC) and ILAC (AIC) international work.
119 participants answered the feedback questionnaire with 98% indicating they were satisfied or very satisfied with this program and the new meeting style. Since the live broadcast, this event has been viewed more than 998 times using the replay function.